Mal-adaptation of event-related EEG responses preceding performance errors
- PMID: 20740080
- PMCID: PMC2927308
- DOI: 10.3389/fnhum.2010.00065
Mal-adaptation of event-related EEG responses preceding performance errors
Abstract
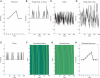
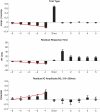
Recent EEG and fMRI evidence suggests that behavioral errors are foreshadowed by systematic changes in brain activity preceding the outcome by seconds. In order to further characterize this type of error precursor activity, we investigated single-trial event-related EEG activity from 70 participants performing a modified Eriksen flanker task, in particular focusing on the trial-by-trial dynamics of a fronto-central independent component that previously has been associated with error and feedback processing. The stimulus-locked peaks in the N2 and P3 latency range in the event-related averages showed expected compatibility and error-related modulations. In addition, a small pre-stimulus negative slow wave was present at erroneous trials. Significant error-preceding activity was found in local stimulus sequences with decreased conflict in the form of less negativity at the N2 latency (310-350 ms) accumulating across five trials before errors; concomitantly response times were speeding across trials. These results illustrate that error-preceding activity in event-related EEG is associated with the performance monitoring system and we conclude that the dynamics of performance monitoring contribute to the generation of error-prone states in addition to the more remote and indirect effects in ongoing activity such as posterior alpha power in EEG and default mode drifts in fMRI.
Keywords: deconvolution; errors; event-related EEG; independent component analysis; performance monitoring; single trial analysis.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials

