Scleraxis expression is coordinately regulated in a murine model of patellar tendon injury
- PMID: 20740671
- PMCID: PMC3951487
- DOI: 10.1002/jor.21220
Scleraxis expression is coordinately regulated in a murine model of patellar tendon injury
Abstract
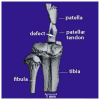
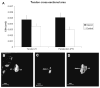
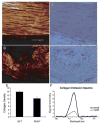
This study investigated the expression of Scleraxis in a murine model of patellar tendon injury in which the central third of the patellar tendon was unilaterally injured. The presence of tendon pathology was assessed using dual photon microscopy, conventional histology and microCT. Tendon pathology was also quantified noninvasively over a 12-week period using high-frequency ultrasound and laser Doppler flowmetry. Gene expression (Scx, Tnmd, and Col1a1) was determined at defined end-points (1, 4, 8, and 12 weeks) using qPCR on RNA from individual patellar tendons on injured and uninjured sides. There was significant development of tendon pathology as gauged by ultrasound and laser Doppler over 12 weeks. Injured tendons demonstrated significant histological and microCT evidence of pathological change, and disorganized collagen with reduced density. The expression of Scx and Col1a1 was unchanged at 1 week, significantly upregulated at 4 and 8 weeks, and had returned to baseline by 12 weeks. Tnmd expression was unchanged at 1 week, and significantly increased at 4, 8, and 12 weeks. Patellar tendon injury was associated with marked increases in the expression of Scx, Tnmd, and Col1a1. Our data suggest new roles for Scleraxis in coordinating the response to injury in the pathogenesis of tendon disorders.
Copyright © 2010 Orthopaedic Research Society.
Figures


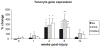
References
-
- Hazleman B, Riley G, Speed CA. Soft tissue rheumatology. Oxford: Oxford University Press; 2004.
-
- Tallon C, Coleman BD, Khan KM, et al. Outcome of surgery for chronic achilles tendinopathy. A critical review. Am J Sports Med. 2001;29:315–320. - PubMed
-
- Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22:675–692. - PubMed
-
- Jarvinen M, Jozsa L, Kannus P, et al. Histopathological findings in chronic tendon disorders. Scand JMed Sci Sports. 1997;7:86–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

