Potent mutagenicity of 3-methylindole requires pulmonary cytochrome P450-mediated bioactivation: a comparison to the prototype cigarette smoke mutagens B(a)P and NNK
- PMID: 20795680
- PMCID: PMC2981624
- DOI: 10.1021/tx100147z
Potent mutagenicity of 3-methylindole requires pulmonary cytochrome P450-mediated bioactivation: a comparison to the prototype cigarette smoke mutagens B(a)P and NNK
Abstract
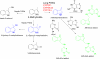
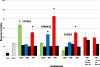
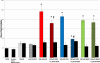
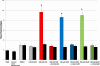
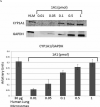
3-Methylindole (3MI) is a preferential pneumotoxicant found in cigarette smoke. A number of lung-expressed human cytochrome P450 enzymes, including 1A1, 2F1, and 2A13, catalyze the metabolism of 3MI to reactive intermediates that fragment DNA, measured with the Comet assay to assess DNA damage, in a cytochrome P450-dependent manner in primary normal human lung cells in culture, but the mutagenesis of 3MI has been controversial. In the present study, the mutagenic potential of 3MI was compared to the prototypical cigarette smoke carcinogens benzo(a)pyrene (B(a)P) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). 3MI, B(a)P, and NNK were incubated with the Salmonella typhimurium strain TA98, which is known to detect the most common subtype of cigarette smoke-induced mutagenicity, frameshift mutations in DNA, and with Salmonella typhimurium strain TA100, which detects base pair substitution mutants, with five sources of P450-mediated bioactivation: rat liver S9, human lung microsomes, recombinant CYP2A13, purified CYP2F3, and recombinant CYP1A1. Only B(a)P was mutagenic in TA100, and it was bioactivated by human lung microsomes and rat liver S9 sources of P450s. However, with the TA98 strain, CYP1A1, CYP2A13, CYP2F3, and human lung microsomes bioactivated 3MI to highly mutagenic intermediates, whereas neither human nor rat liver S9 subcellular fractions formed mutagenic intermediates from 3MI. Quantitative Western blot analysis verified that all three respiratory enzymes were present in human lung microsomes in widely varying amounts. These results indicate that metabolism of 3MI by human lung-expressed cytochrome P450 enzymes but not hepatic P450s elicits equivalent or higher mutagenicity than the prototype cigarette smoke mutagens B(a)P and NNK and indicates that 3MI is a likely human pulmonary carcinogen.
Figures




Similar articles
-
3-Methylindole is mutagenic and a possible pulmonary carcinogen.Toxicol Sci. 2009 Nov;112(1):59-67. doi: 10.1093/toxsci/kfp201. Epub 2009 Aug 21. Toxicol Sci. 2009. PMID: 19700606 Free PMC article.
-
3-Methylindole metabolites induce lung CYP1A1 and CYP2F1 enzymes by AhR and non-AhR mechanisms, respectively.Chem Res Toxicol. 2010 Mar 15;23(3):696-704. doi: 10.1021/tx9004506. Chem Res Toxicol. 2010. PMID: 20187624 Free PMC article.
-
Comparative metabolism of the tobacco-specific nitrosamines 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol by rat cytochrome P450 2A3 and human cytochrome P450 2A13.Drug Metab Dispos. 2003 Oct;31(10):1199-202. doi: 10.1124/dmd.31.10.1199. Drug Metab Dispos. 2003. PMID: 12975327
-
Activation and effects of the food-derived heterocyclic amines in extrahepatic tissues.Princess Takamatsu Symp. 1995;23:123-33. Princess Takamatsu Symp. 1995. PMID: 8844803 Review.
-
The role of cytochrome P450 enzymes in carcinogen activation and detoxication: an in vivo-in vitro paradox.Carcinogenesis. 2018 Jul 3;39(7):851-859. doi: 10.1093/carcin/bgy058. Carcinogenesis. 2018. PMID: 29726902 Free PMC article. Review.
Cited by
-
Generation and characterization of a CYP2A13/2B6/2F1-transgenic mouse model.Drug Metab Dispos. 2012 Jun;40(6):1144-50. doi: 10.1124/dmd.112.044826. Epub 2012 Mar 7. Drug Metab Dispos. 2012. PMID: 22397853 Free PMC article.
-
Nicotine Component of Cigarette Smoke Extract (CSE) Decreases the Cytotoxicity of CSE in BEAS-2B Cells Stably Expressing Human Cytochrome P450 2A13.Int J Environ Res Public Health. 2017 Oct 13;14(10):1221. doi: 10.3390/ijerph14101221. Int J Environ Res Public Health. 2017. PMID: 29027939 Free PMC article.
-
Human Family 1-4 cytochrome P450 enzymes involved in the metabolic activation of xenobiotic and physiological chemicals: an update.Arch Toxicol. 2021 Feb;95(2):395-472. doi: 10.1007/s00204-020-02971-4. Epub 2021 Jan 18. Arch Toxicol. 2021. PMID: 33459808 Free PMC article. Review.
-
Respective roles of CYP2A5 and CYP2F2 in the bioactivation of 3-methylindole in mouse olfactory mucosa and lung: studies using Cyp2a5-null and Cyp2f2-null mouse models.Drug Metab Dispos. 2012 Apr;40(4):642-7. doi: 10.1124/dmd.111.044081. Epub 2012 Jan 6. Drug Metab Dispos. 2012. PMID: 22228748 Free PMC article.
-
Metabolomics Provides Novel Insights into the Potential Toxicity Associated with Heated Tobacco Products, Electronic Cigarettes, and Tobacco Cigarettes on Human Bronchial Epithelial BEAS-2B Cells.Toxics. 2024 Feb 4;12(2):128. doi: 10.3390/toxics12020128. Toxics. 2024. PMID: 38393223 Free PMC article.
References
-
- Smith CJ, Perfetti TA, Garg R, Hansch C. IARC carcinogens reported in cigarette mainstream smoke and their calculated log P values. Food Chem Toxicol. 2003;41:807–817. - PubMed
-
- Hoffmann D, Hoffmann I, El-Bayoumy K. The less harmful cigarette: a controversial issue. a tribute to Ernst L. Wynder. Chem Res Toxicol. 2001;14:767–790. - PubMed
-
- DeMarini DM, Gudi R, Szkudlinska A, Rao M, Recio L, Kehl M, Kirby PE, Polzin G, Richter PA. Genotoxicity of 10 cigarette smoke condensates in four test systems: comparisons between assays and condensates. Mutat Res. 2008;650:15–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

