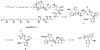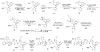Synthesis of the bis-tetrahydropyran core of amphidinol 3
- PMID: 20795745
- PMCID: PMC2929927
- DOI: 10.1021/ol1015898
Synthesis of the bis-tetrahydropyran core of amphidinol 3
Abstract
A convergent synthesis of the C31-C52 bis-tetrahydropyran core of the natural product amphidinol 3 is reported. A common intermediate was synthesized from d-tartaric acid utilizing an asymmetric glycolate alkylation/ring-closing metathesis sequence to construct the THP rings. Differential elaboration of the common intermediate allowed the synthesis of two distinct coupling partners which were joined through a modified Horner-Wadsworth-Emmons olefination to provide the bis-tetrahydropyran core.
Figures
References
-
- Satake M, Murata M, Yasumoto T, Fujita T, Naoki H. J Am Chem Soc. 1991;113:9859–9861.
-
- Paul GK, Matsumori N, Konoki K, Sasaki M, Murata M, Tachibana K. Harmful and Toxic Algal Blooms
-
- Murata M, Matsuoka S, Matsumori N, Paul GK, Tachibana K. J Am Chem Soc. 1999;121:870.
-
- Oishi T, Kanemoto M, Swasono R, Matsumori N, Murata M. Org Lett. 2008;10:5203–5206. - PubMed
-
- BouzBouz S, Cossy J. Org Lett. 2001;3:1451–1454. - PubMed
- Cossy J, Tsuchiya T, Ferrie L, Reymond S, Kreuzer T, Colobert F, Jourdain P, Marko IE. Synlett. 2007:2286–2288.
- Colobert F, Kreuzer T, Cossy J, Reymond S, Tsuchiya T, Ferrie L, Marko IE, Jourdain P. Synlett. 2007:2351–2354.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous