Apatite microtopographies instruct signaling tapestries for progenitor-driven new attachment of teeth
- PMID: 20795795
- PMCID: PMC3029000
- DOI: 10.1089/ten.TEA.2010.0264
Apatite microtopographies instruct signaling tapestries for progenitor-driven new attachment of teeth
Abstract
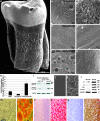
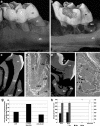
Dimension and structure of extracellular matrix surfaces have powerful influences on cell shape, adhesion, and gene expression. Here we show that natural tooth root topographies induce integrin-mediated extracellular matrix signaling cascades in tandem with cell elongation and polarization to generate physiological periodontium-like tissues. In this study we replanted surface topography instructed periodontal progenitors into rat alveolar bone sockets for 8 and 16 weeks, resulting in complete reattachment of tooth roots to the surrounding alveolar bone with a periodontal fiber apparatus closely matching physiological controls along the entire root surface. Displacement studies and biochemical analyses confirmed that progenitor-based engineered periodontal tissues were similar to control teeth and uniquely derived from preimplantation green fluorescent protein (GFP)-labeled progenitors. Together, these studies illustrate the capacity of natural extracellular surface topographies to instruct progenitor cell populations to fully regenerate complex cellular and structural morphologies of tissues once lost to disease. We suggest that our strategy could be used for the replantation of teeth lost due to trauma or as a novel approach for tooth replacement using tooth-shaped replicas.
Figures



Similar articles
-
Successful periodontal ligament regeneration by periodontal progenitor preseeding on natural tooth root surfaces.Stem Cells Dev. 2011 Oct;20(10):1659-68. doi: 10.1089/scd.2010.0431. Epub 2011 Mar 9. Stem Cells Dev. 2011. PMID: 21250830 Free PMC article.
-
Periodontal regeneration with stem cells-seeded collagen-hydroxyapatite scaffold.J Biomater Appl. 2016 Jul;31(1):121-31. doi: 10.1177/0885328216637978. Epub 2016 Mar 22. J Biomater Appl. 2016. PMID: 27009932
-
Developmental pathways of periodontal tissue regeneration: Developmental diversities of tooth morphogenesis do also map capacity of periodontal tissue regeneration?J Periodontal Res. 2019 Feb;54(1):10-26. doi: 10.1111/jre.12596. Epub 2018 Sep 12. J Periodontal Res. 2019. PMID: 30207395 Review.
-
Experimental formation of periodontal structure around titanium implants utilizing bone marrow mesenchymal stem cells: a pilot study.J Oral Implantol. 2009;35(3):106-29. doi: 10.1563/1548-1336-35.3.106. J Oral Implantol. 2009. PMID: 19579523
-
The application of bone morphogenetic proteins to dental tissue engineering.Nat Biotechnol. 2003 Sep;21(9):1025-32. doi: 10.1038/nbt864. Nat Biotechnol. 2003. PMID: 12949568 Review.
Cited by
-
SCF promotes dental pulp progenitor migration, neovascularization, and collagen remodeling - potential applications as a homing factor in dental pulp regeneration.Stem Cell Rev Rep. 2013 Oct;9(5):655-67. doi: 10.1007/s12015-013-9442-7. Stem Cell Rev Rep. 2013. PMID: 23703692 Free PMC article.
-
Lyophilized platelet-rich fibrin (PRF) promotes craniofacial bone regeneration through Runx2.Int J Mol Sci. 2014 May 14;15(5):8509-25. doi: 10.3390/ijms15058509. Int J Mol Sci. 2014. PMID: 24830554 Free PMC article.
-
In vitro evaluation of surface roughness, adhesion of periodontal ligament fibroblasts, and Streptococcus gordonii following root instrumentation with Gracey curettes and subsequent polishing with diamond-coated curettes.Clin Oral Investig. 2013 Mar;17(2):397-404. doi: 10.1007/s00784-012-0719-z. Epub 2012 Apr 17. Clin Oral Investig. 2013. PMID: 22526889
-
MicroRNAs and Periodontal Homeostasis.J Dent Res. 2017 May;96(5):491-500. doi: 10.1177/0022034516685711. Epub 2017 Jan 9. J Dent Res. 2017. PMID: 28068481 Free PMC article. Review.
-
Histone Methylation Mechanisms Modulate the Inflammatory Response of Periodontal Ligament Progenitors.Stem Cells Dev. 2019 Aug 1;28(15):1015-1025. doi: 10.1089/scd.2019.0125. Epub 2019 Jul 22. Stem Cells Dev. 2019. PMID: 31218921 Free PMC article.
References
-
- Tan W. Desai T.A. Microfluidic patterning of cells in extracellular matrix biopolymers: effects of channel size, cell type, and matrix composition on pattern integrity. Tissue Eng. 2003;9:255. - PubMed
-
- Norman J.J. Desai T.A. Methods for fabrication of nanoscale topography for tissue engineering scaffolds. Ann Biomed Eng. 2006;34:89. - PubMed
-
- Matsuura M. Herr Y. Han K.Y. Lin W.L. Genco R.J. Cho M. I Immunohistochemical expression of extracellular matrix components of normal, healing periodontal tissues in the beagle dog. J Periodontol. 1995;66:579. - PubMed
-
- Waddington R.J. Embery G. Proteoglycans and orthodontic tooth movement. J Orthod. 2001;28:281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

