MMP-7 knock-in corneal fibroblast cell lines secrete MMP-7 with proteolytic activity towards collagen XVIII
- PMID: 20795861
- PMCID: PMC3678734
- DOI: 10.3109/02713683.2010.494239
MMP-7 knock-in corneal fibroblast cell lines secrete MMP-7 with proteolytic activity towards collagen XVIII
Abstract
Purpose: To determine whether matrix metalloproteinase-7 (MMP-7) that is stably overexpressed by mouse corneal fibroblast cell lines exhibits proteolytic activity against the NC1 fragment of collagen XVIII.
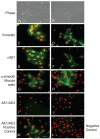
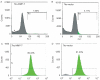
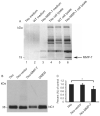
Methods: Corneal fibroblasts isolated from MMP-7 knockout (7ko) mice were subjected to SV40 T-antigen immortalization and stably transfected with a bicistronic retroviral vector encoding green fluorescence protein and active MMP-7. The resulting MMP-7 knock-in fibroblasts (7ko-MMP-7 cells) were isolated and enriched by fluorescence activated cell sorting (FACS). Culture media samples from 7ko and 7ko-MMP-7 cells were then incubated with the recombinant NC1 fragment of collagen XVIII, and NC1 degradation was monitored by immunoblotting.
Results: Immunoblot analysis revealed that MMP-7 was present in lysates and culture media from 7ko-MMP-7 fibroblasts, but not media from immortalized 7ko fibroblasts. Importantly, lower amounts of the NC1 fragment were present in in vitro enzymatic reaction mixtures containing concentrated 7ko-MMP-7 media than in those containing concentrated 7ko media.
Conclusion: Immortalized fibroblasts stably transfected with MMP-7 secrete active MMP-7 with proteolytic activity towards the NC1 fragment of collagen XVIII.
Figures


References
-
- Daniels JT, Cambrey AD, Occleston NL, et al. Matrix metalloproteinase inhibition modulates fibroblast-mediated matrix contraction and collagen production in vitro. Invest Ophthalmol Vis Sci. 2003;44:1104–1110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
