Insights into the neurodevelopmental origin of schizophrenia from postmortem studies of prefrontal cortical circuitry
- PMID: 20797429
- PMCID: PMC3319737
- DOI: 10.1016/j.ijdevneu.2010.08.003
Insights into the neurodevelopmental origin of schizophrenia from postmortem studies of prefrontal cortical circuitry
Abstract
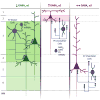
The hypothesis that schizophrenia results from a developmental, as opposed to a degenerative, process affecting the connectivity and network plasticity of the cerebral cortex is supported by findings from morphological and molecular postmortem studies. Specifically, abnormalities in the expression of protein markers of GABA neurotransmission and the lamina- and circuit-specificity of these changes in the cortex in schizophrenia, in concert with knowledge of their developmental trajectories, offer crucial insight into the vulnerability of specific cortical networks to environmental insults during different periods of development. These findings reveal potential targets for therapeutic interventions to improve cognitive function in individuals with schizophrenia, and provide guidance for future preventive strategies to preserve cortical neurotransmission in at-risk individuals.
Copyright © 2010 ISDN. Published by Elsevier Ltd. All rights reserved.
Figures


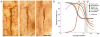


References
-
- Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Rev. 2006;52:293–304. - PubMed
-
- Akbarian S, Huntsman MM, Kim JJ, Tafazzoli A, Potkin SG, Bunney WE, Jr, Jones EG. GABAA receptor subunit gene expression in human prefrontal cortex: comparison of schizophrenics and controls. Cereb Cortex. 1995a;5:550–560. - PubMed
-
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995b;52:258–266. - PubMed
-
- Alexander GE, Goldman PS. Functional development of the dorsolateral prefrontal cortex: an analysis utlizing reversible cryogenic depression. Brain Res. 1978;143:233–249. - PubMed
-
- Anderson SA, Classey JD, Conde F, Lund JS, Lewis DA. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67:7–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

