Recruitment of infants with sickle cell anemia to a Phase III trial: data from the BABY HUG study
- PMID: 20797449
- PMCID: PMC2975355
- DOI: 10.1016/j.cct.2010.08.007
Recruitment of infants with sickle cell anemia to a Phase III trial: data from the BABY HUG study
Abstract
Background: Protocol-eligible subjects may not be candidates for research participation or may decline. To determine factors that affected accrual, we evaluated enrollment in BABY HUG, a multi-center, randomized, placebo-controlled Phase III trial of hydroxyurea (HU) in infants with sickle cell anemia.
Methods: An anonymized registry of potential subjects served as the primary source of data. Study coordinators considered all infants less than age 18 months with a hemoglobin FS diagnosis on newborn screening. Data included the number of potentially eligible subjects, whether parents were approached, and reasons for participating or declining.
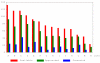
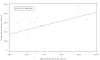
Results: Of 1106 potential participants, 28% were not approached for reasons such as prior poor adherence to clinical care. Interested families expressed willingness to contribute to medical knowledge (51%), hope of being randomized to receive hydroxyurea (51%), and desire for closer clinical care (51%) as reasons for participating. Disease severity or the perception that their child was ill had less impact on willingness to participate (16%). Parents who declined cited fear of research (19%), transportation problems (14%), and the demanding nature of the study (25%). Ultimately, 234 (21%) gave informed consent, with little variability of acceptance rates among sites. Importantly, the number of subjects enrolled correlated with the number of families that were approached. Sites that excluded patients based on clinical/psychosocial biases were not more successful in recruiting than those who approached all eligible subjects.
Conclusion: Large, demanding clinical trials require an adequate pool of potential participants. Approaching all potentially eligible patients without predetermined biases enhances success in recruitment.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
References
-
- Gross D. A research agenda for understanding particpation in clinical research. Research in Nursing & Health. 2006;29:172–5. - PubMed
-
- Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al. Recruitment to randomized trials: stategies for trial enrollment and particpation study. The STEPS study. Health Technol Assess. 2007;11:48. - PubMed
-
- Wang WC. Sickle cell anemia and other sickling syndromes (Chpt 37) In: Greer JP, Foerster J, Rodgers GM, Paraskevas F, Glader BE, editors. Wintrobe’s Clinical Hematology. 12th edition Lippincott Williams and Wilkins; Philadelphia: 2008. pp. 1038–1082.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases