Prevalence and predictors of off-label use of cardiac resynchronization therapy in patients enrolled in the National Cardiovascular Data Registry Implantable Cardiac-Defibrillator Registry
- PMID: 20797489
- PMCID: PMC2958057
- DOI: 10.1016/j.jacc.2010.05.025
Prevalence and predictors of off-label use of cardiac resynchronization therapy in patients enrolled in the National Cardiovascular Data Registry Implantable Cardiac-Defibrillator Registry
Abstract
Objectives: The purpose of the study was to define the extent and nature of cardiac resynchronization therapy (CRT) device usage outside consensus guidelines using national data.
Background: Recent literature has shown that the application of CRT in clinical practice frequently does not adhere to evidence-based consensus guidelines. Factors underlying these practices have not been fully explored.
Methods: From the National Cardiovascular Data Registry's Implantable Cardiac-Defibrillator Registry, we defined a cohort of 45,392 cardiac resynchronization therapy-defibrillator (CRT-D) implants between January 2006 and June 2008 with a primary prevention indication. We defined "off-label" implants as those in which the ejection fraction was >35%, the New York Heart Association functional class was below III, or the QRS interval duration was <120 ms in the absence of a documented need for ventricular pacing. The relationships between patient, implanting physician, and hospital characteristics with off-label use were explored with multivariable hierarchical logistic regression models.
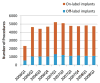
Results: Overall, 23.7% of devices were placed without meeting all 3 implant criteria, most often due to New York Heart Association functional class below III (13.1% of implants) or QRS interval duration <120 ms (12.0%). Atrial fibrillation/flutter, previous percutaneous coronary intervention, and the performance of an electrophysiology study before implant were independently associated with increased odds of off-label use, whereas diabetes mellitus, increasing age, and female sex were associated with decreased odds. Physician training and insurance payer were weakly associated with the likelihood of off-label use.
Conclusions: Nearly 1 in 4 patients receiving CRT devices in the study time frame did not meet guideline-based indications. Given the evolving evidence base supporting the use of CRT, these practices require careful scrutiny.
Copyright (c) 2010 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Use of Cardiac Resynchronization Therapy Among Eligible Patients Receiving an Implantable Cardioverter Defibrillator: Insights From the National Cardiovascular Data Registry Implantable Cardioverter Defibrillator Registry.JAMA Cardiol. 2017 May 1;2(5):561-565. doi: 10.1001/jamacardio.2016.5388. JAMA Cardiol. 2017. PMID: 28122073 Free PMC article.
-
Use and Outcomes of Dual Chamber or Cardiac Resynchronization Therapy Defibrillators Among Older Patients Requiring Ventricular Pacing in the National Cardiovascular Data Registry Implantable Cardioverter Defibrillator Registry.JAMA Netw Open. 2021 Jan 4;4(1):e2035470. doi: 10.1001/jamanetworkopen.2020.35470. JAMA Netw Open. 2021. PMID: 33496796 Free PMC article.
-
Comparative Effectiveness of Cardiac Resynchronization Therapy Among Patients With Heart Failure and Atrial Fibrillation: Findings From the National Cardiovascular Data Registry's Implantable Cardioverter-Defibrillator Registry.Circ Heart Fail. 2016 Jun;9(6):10.1161/CIRCHEARTFAILURE.115.002324 e002324. doi: 10.1161/CIRCHEARTFAILURE.115.002324. Circ Heart Fail. 2016. PMID: 27296396 Free PMC article.
-
Cardiac resynchronization therapy and implantable cardiac defibrillators in left ventricular systolic dysfunction.Evid Rep Technol Assess (Full Rep). 2007 Jun;(152):1-199. Evid Rep Technol Assess (Full Rep). 2007. PMID: 17764218 Free PMC article. Review.
-
Effectiveness of implantable cardioverter defibrillators and cardiac resynchronization therapy in heart failure.Heart Fail Clin. 2013 Jan;9(1):59-77. doi: 10.1016/j.hfc.2012.09.006. Epub 2012 Oct 13. Heart Fail Clin. 2013. PMID: 23168318 Review.
Cited by
-
Cardiac Resynchronization Therapy in Patients with Atrial Fibrillation - Worth the Effort?J Atr Fibrillation. 2012 Apr 14;4(6):435. doi: 10.4022/jafib.435. eCollection 2012 Apr-May. J Atr Fibrillation. 2012. PMID: 28496729 Free PMC article. Review.
-
Temporal trends in quality of care among recipients of implantable cardioverter-defibrillators: insights from the National Cardiovascular Data Registry.Circulation. 2014 Feb 4;129(5):580-6. doi: 10.1161/CIRCULATIONAHA.113.003747. Epub 2013 Nov 5. Circulation. 2014. PMID: 24192798 Free PMC article.
-
Mortality risk following replacement implantable cardioverter-defibrillator implantation at end of battery life: results from the NCDR.Heart Rhythm. 2014 Feb;11(2):216-21. doi: 10.1016/j.hrthm.2013.10.046. Heart Rhythm. 2014. PMID: 24513917 Free PMC article.
-
Prevalence of guideline-directed medical therapy among patients receiving cardiac resynchronization therapy defibrillator implantation in the National Cardiovascular Data Registry during the years 2006 to 2008.Am J Cardiol. 2014 Jun 15;113(12):2052-6. doi: 10.1016/j.amjcard.2014.03.049. Epub 2014 Apr 1. Am J Cardiol. 2014. PMID: 24793671 Free PMC article.
-
Medical device postapproval safety monitoring: where does the United States stand?Circ Cardiovasc Qual Outcomes. 2015 Jan;8(1):124-31. doi: 10.1161/CIRCOUTCOMES.114.001460. Epub 2015 Jan 6. Circ Cardiovasc Qual Outcomes. 2015. PMID: 25563662 Free PMC article. No abstract available.
References
-
- Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. - PubMed
-
- Cleland JG, Daubert JC, Erdmann E, et al. The CARE-HF study (CArdiac REsynchronisation in Heart Failure Study): rationale, design and end-points. Eur J Heart Fail. 2001;3:481–9. - PubMed
-
- Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. - PubMed
-
- Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. - PubMed
-
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–82. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

