Myosin IIb regulates actin dynamics during synaptic plasticity and memory formation
- PMID: 20797537
- PMCID: PMC2929390
- DOI: 10.1016/j.neuron.2010.07.016
Myosin IIb regulates actin dynamics during synaptic plasticity and memory formation
Abstract
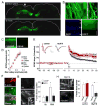
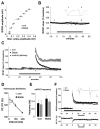
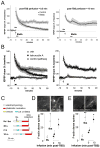
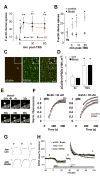
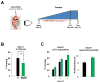
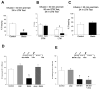
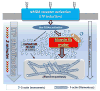
Reorganization of the actin cytoskeleton is essential for synaptic plasticity and memory formation. Presently, the mechanisms that trigger actin dynamics during these brain processes are poorly understood. In this study, we show that myosin II motor activity is downstream of LTP induction and is necessary for the emergence of specialized actin structures that stabilize an early phase of LTP. We also demonstrate that myosin II activity contributes importantly to an actin-dependent process that underlies memory consolidation. Pharmacological treatments that promote actin polymerization reversed the effects of a myosin II inhibitor on LTP and memory. We conclude that myosin II motors regulate plasticity by imparting mechanical forces onto the spine actin cytoskeleton in response to synaptic stimulation. These cytoskeletal forces trigger the emergence of actin structures that stabilize synaptic plasticity. Our studies provide a mechanical framework for understanding cytoskeletal dynamics associated with synaptic plasticity and memory formation.
2010 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Myosin IIb-dependent Regulation of Actin Dynamics Is Required for N-Methyl-D-aspartate Receptor Trafficking during Synaptic Plasticity.J Biol Chem. 2015 Oct 16;290(42):25395-410. doi: 10.1074/jbc.M115.644229. Epub 2015 Sep 1. J Biol Chem. 2015. PMID: 26330558 Free PMC article.
-
Regulation of synapse structure and function by distinct myosin II motors.J Neurosci. 2011 Jan 26;31(4):1448-60. doi: 10.1523/JNEUROSCI.3294-10.2011. J Neurosci. 2011. PMID: 21273429 Free PMC article.
-
Myosin II motor activity in the lateral amygdala is required for fear memory consolidation.Learn Mem. 2011 Dec 14;19(1):9-14. doi: 10.1101/lm.024042.111. Print 2012 Jan. Learn Mem. 2011. PMID: 22174310 Free PMC article.
-
Regulation of neuronal PKA signaling through AKAP targeting dynamics.Eur J Cell Biol. 2006 Jul;85(7):627-33. doi: 10.1016/j.ejcb.2006.01.010. Epub 2006 Feb 28. Eur J Cell Biol. 2006. PMID: 16504338 Review.
-
Role of Drebrin in Synaptic Plasticity.Adv Exp Med Biol. 2017;1006:183-201. doi: 10.1007/978-4-431-56550-5_11. Adv Exp Med Biol. 2017. PMID: 28865021 Review.
Cited by
-
Sex differences in synaptic plasticity underlying learning.J Neurosci Res. 2023 May;101(5):764-782. doi: 10.1002/jnr.24844. Epub 2021 Apr 12. J Neurosci Res. 2023. PMID: 33847004 Free PMC article. Review.
-
Spine dynamics in the brain, mental disorders and artificial neural networks.Nat Rev Neurosci. 2021 Jul;22(7):407-422. doi: 10.1038/s41583-021-00467-3. Epub 2021 May 28. Nat Rev Neurosci. 2021. PMID: 34050339 Review.
-
PKMζ knockdown disrupts post-ischemic long-term potentiation via inhibiting postsynaptic expression of aminomethyl phosphonic acid receptors.J Biomed Res. 2015 May;29(3):241-9. doi: 10.7555/JBR.28.20140033. Epub 2014 Jun 18. J Biomed Res. 2015. PMID: 26060448 Free PMC article. Review.
-
State-dependent diffusion of actin-depolymerizing factor/cofilin underlies the enlargement and shrinkage of dendritic spines.Sci Rep. 2016 Sep 6;6:32897. doi: 10.1038/srep32897. Sci Rep. 2016. PMID: 27595610 Free PMC article.
-
Conventional and Non-Conventional Roles of Non-Muscle Myosin II-Actin in Neuronal Development and Degeneration.Cells. 2020 Aug 19;9(9):1926. doi: 10.3390/cells9091926. Cells. 2020. PMID: 32825197 Free PMC article. Review.
References
-
- Backouche F, Haviv L, Groswasser D, Bernheim-Groswasser A. Active gels: dynamics of patterning and self-organization. Phys Biol. 2006;3:264–273. - PubMed
-
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzyczka N. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R00 DA024761/DA/NIDA NIH HHS/United States
- R01 NS064079/NS/NINDS NIH HHS/United States
- NS064079/NS/NINDS NIH HHS/United States
- F31 MH083396/MH/NIMH NIH HHS/United States
- MH083396/MH/NIMH NIH HHS/United States
- T32 AG000096/AG/NIA NIH HHS/United States
- P01 HL059412/HL/NHLBI NIH HHS/United States
- NS45260/NS/NINDS NIH HHS/United States
- NS05182/NS/NINDS NIH HHS/United States
- AG00096/AG/NIA NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- NINDS 045540/PHS HHS/United States
- P01 NS045260/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

