Functional studies of Plasmodium falciparum dipeptidyl aminopeptidase I using small molecule inhibitors and active site probes
- PMID: 20797610
- PMCID: PMC2929396
- DOI: 10.1016/j.chembiol.2010.06.007
Functional studies of Plasmodium falciparum dipeptidyl aminopeptidase I using small molecule inhibitors and active site probes
Abstract
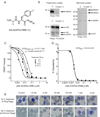
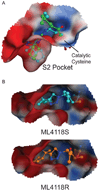
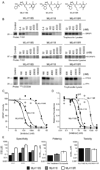
The widespread resistance of malaria parasites to all affordable drugs has made the identification of new targets urgent. Dipeptidyl aminopeptidases (DPAPs) represent potentially valuable new targets that are involved in hemoglobin degradation (DPAP1) and parasite egress (DPAP3). Here we use activity-based probes to demonstrate that specific inhibition of DPAP1 by a small molecule results in the formation of an immature trophozoite that leads to parasite death. Using computational methods, we designed stable, nonpeptidic covalent inhibitors that kill Plasmodium falciparum at low nanomolar concentrations. These compounds show signs of slowing parasite growth in a murine model of malaria, which suggests that DPAP1 might be a viable antimalarial target. Interestingly, we found that resynthesis and activation of DPAP1 after inhibition is rapid, suggesting that effective drugs would need to sustain DPAP1 inhibition for a period of 2-3 hr.
Copyright (c) 2010 Elsevier Ltd. All rights reserved.
Figures

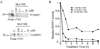

Similar articles
-
Biochemical characterization of Plasmodium falciparum dipeptidyl aminopeptidase 1.Mol Biochem Parasitol. 2011 Jan;175(1):10-20. doi: 10.1016/j.molbiopara.2010.08.004. Epub 2010 Sep 15. Mol Biochem Parasitol. 2011. PMID: 20833209 Free PMC article.
-
A Plasmodium falciparum dipeptidyl aminopeptidase I participates in vacuolar hemoglobin degradation.J Biol Chem. 2004 Oct 8;279(41):43000-7. doi: 10.1074/jbc.M408123200. Epub 2004 Aug 10. J Biol Chem. 2004. PMID: 15304495
-
Characterization of P. falciparum dipeptidyl aminopeptidase 3 specificity identifies differences in amino acid preferences between peptide-based substrates and covalent inhibitors.FEBS J. 2019 Oct;286(20):3998-4023. doi: 10.1111/febs.14953. Epub 2019 Jun 24. FEBS J. 2019. PMID: 31177613 Free PMC article.
-
Proteases of malaria parasites: new targets for chemotherapy.Emerg Infect Dis. 1998 Jan-Mar;4(1):49-57. doi: 10.3201/eid0401.980107. Emerg Infect Dis. 1998. PMID: 9452398 Free PMC article. Review.
-
Aminopeptidases of malaria parasites: new targets for chemotherapy.Infect Disord Drug Targets. 2010 Jun;10(3):217-25. doi: 10.2174/187152610791163363. Infect Disord Drug Targets. 2010. PMID: 20334618 Review.
Cited by
-
Identification of Plasmodium dipeptidyl aminopeptidase allosteric inhibitors by high throughput screening.PLoS One. 2019 Dec 18;14(12):e0226270. doi: 10.1371/journal.pone.0226270. eCollection 2019. PLoS One. 2019. PMID: 31851699 Free PMC article.
-
Structural Insights Into Key Plasmodium Proteases as Therapeutic Drug Targets.Front Microbiol. 2019 Mar 5;10:394. doi: 10.3389/fmicb.2019.00394. eCollection 2019. Front Microbiol. 2019. PMID: 30891019 Free PMC article. Review.
-
A research agenda for malaria eradication: basic science and enabling technologies.PLoS Med. 2011 Jan 25;8(1):e1000399. doi: 10.1371/journal.pmed.1000399. PLoS Med. 2011. PMID: 21311584 Free PMC article. Review.
-
System-wide biochemical analysis reveals ozonide antimalarials initially act by disrupting Plasmodium falciparum haemoglobin digestion.PLoS Pathog. 2020 Jun 26;16(6):e1008485. doi: 10.1371/journal.ppat.1008485. eCollection 2020 Jun. PLoS Pathog. 2020. PMID: 32589689 Free PMC article.
-
Drug delivery to the malaria parasite using an arterolane-like scaffold.ChemMedChem. 2015 Jan;10(1):47-51. doi: 10.1002/cmdc.201402362. Epub 2014 Oct 14. ChemMedChem. 2015. PMID: 25314098 Free PMC article.
References
-
- Arastu-Kapur S, Ponder EL, Fonovic UP, Yeoh S, Yuan F, Fonovic M, Grainger M, Phillips CI, Powers JC, Bogyo M. Identification of proteases that regulate erythrocyte rupture by the malaria parasite Plasmodium falciparum. Nature chemical biology. 2008;4:203–213. - PubMed
-
- Aregawi M, Cibulskis R, Otten M, Williams R, Dye C, Programme WGM. World Malaria Report 2008. World Health Organization; 2008. p. 190.
-
- Binder EM, Kim K. Location, location, location: trafficking and function of secreted proteases of Toxoplasma and Plasmodium. Traffic. 2004;5:914–924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

