Structure of cytochrome P450 PimD suggests epoxidation of the polyene macrolide pimaricin occurs via a hydroperoxoferric intermediate
- PMID: 20797613
- PMCID: PMC2932657
- DOI: 10.1016/j.chembiol.2010.05.026
Structure of cytochrome P450 PimD suggests epoxidation of the polyene macrolide pimaricin occurs via a hydroperoxoferric intermediate
Abstract
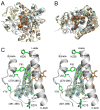
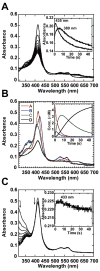
We present the X-ray structure of PimD, both substrate-free and in complex with 4,5-desepoxypimaricin. PimD is a cytochrome P450 monooxygenase with native epoxidase activity that is critical in the biosynthesis of the polyene macrolide antibiotic pimaricin. Intervention in this secondary metabolic pathway could advance the development of drugs with improved pharmacologic properties. Epoxidation by P450 typically includes formation of a charge-transfer complex between an oxoferryl pi-cation radical species (Compound I) and the olefin pi-bond as the initial intermediate. Catalytic and structural evidence presented here suggest that epoxidation of 4,5-desepoxypimaricin proceeds via a hydroperoxoferric intermediate (Compound 0). The oxygen atom of Compound 0 distal to the heme iron may insert into the double bond of the substrate to make an epoxide ring. Stereoelectronic features of the putative transition state suggest substrate-assisted proton delivery.
Copyright (c) 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures


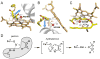

Similar articles
-
Characterization of the polyene macrolide P450 epoxidase from Streptomyces natalensis that converts de-epoxypimaricin into pimaricin.Biochem J. 2005 Feb 15;386(Pt 1):57-62. doi: 10.1042/BJ20040490. Biochem J. 2005. PMID: 15228385 Free PMC article.
-
A proton-shuttle mechanism mediated by the porphyrin in benzene hydroxylation by cytochrome p450 enzymes.J Am Chem Soc. 2003 Jun 18;125(24):7413-24. doi: 10.1021/ja034142f. J Am Chem Soc. 2003. PMID: 12797816
-
Structural analysis of P450 AmphL from Streptomyces nodosus provides insights into substrate selectivity of polyene macrolide antibiotic biosynthetic P450s.J Biol Chem. 2022 Apr;298(4):101746. doi: 10.1016/j.jbc.2022.101746. Epub 2022 Feb 18. J Biol Chem. 2022. PMID: 35189143 Free PMC article.
-
Hydroperoxoferric heme intermediate as a second electrophilic oxidant in cytochrome P450-catalyzed reactions.J Biol Inorg Chem. 2004 Sep;9(6):644-53. doi: 10.1007/s00775-004-0575-7. Epub 2004 Jul 29. J Biol Inorg Chem. 2004. PMID: 15365901 Review.
-
Updating the Paradigm: Redox Partner Binding and Conformational Dynamics in Cytochromes P450.Acc Chem Res. 2022 Feb 1;55(3):373-380. doi: 10.1021/acs.accounts.1c00632. Epub 2021 Dec 29. Acc Chem Res. 2022. PMID: 34965086 Free PMC article. Review.
Cited by
-
The important role of P450 monooxygenase for the biosynthesis of new benzophenones from Cytospora rhizophorae.Appl Microbiol Biotechnol. 2021 Dec;105(24):9219-9230. doi: 10.1007/s00253-021-11648-6. Epub 2021 Nov 22. Appl Microbiol Biotechnol. 2021. PMID: 34807300
-
Proximal ligand electron donation and reactivity of the cytochrome P450 ferric-peroxo anion.J Am Chem Soc. 2012 Apr 18;134(15):6673-84. doi: 10.1021/ja211499q. Epub 2012 Apr 4. J Am Chem Soc. 2012. PMID: 22444582 Free PMC article.
-
Diversity of P450 enzymes in the biosynthesis of natural products.Nat Prod Rep. 2012 Oct;29(10):1251-66. doi: 10.1039/c2np20020a. Epub 2012 Jul 23. Nat Prod Rep. 2012. PMID: 22820933 Free PMC article. Review.
-
Molecular control of polyene macrolide biosynthesis: direct binding of the regulator PimM to eight promoters of pimaricin genes and identification of binding boxes.J Biol Chem. 2011 Mar 18;286(11):9150-61. doi: 10.1074/jbc.M110.182428. Epub 2010 Dec 27. J Biol Chem. 2011. PMID: 21187288 Free PMC article.
-
Investigating the Active Oxidants Involved in Cytochrome P450 Catalyzed Sulfoxidation Reactions.Chemistry. 2022 Dec 27;28(72):e202202428. doi: 10.1002/chem.202202428. Epub 2022 Nov 9. Chemistry. 2022. PMID: 36169207 Free PMC article.
References
-
- Collaborative Computational Project Number 4. Acta Crysallogr D. 1994;50:760–763. - PubMed
-
- Aparicio JF, Caffrey P, Gil JA, Zotchev SB. Polyene antibiotic biosynthesis gene clusters. Appl Microbiol Biotechnol. 2003;61:179–188. - PubMed
-
- Aparicio JF, Mendes MV, Anton N, Recio E, Martin JF. Polyene macrolide antibiotic biosynthesis. Curr Med Chem. 2004;11:1645–1656. - PubMed
-
- Bach RD, Dmitrenko O. Strain energy of small ring hydrocarbons. Influence of C-H bond dissociation energies. J Am Chem Soc. 2004;126:4444–4452. - PubMed
-
- Baginski M, Czub J, Sternal K. Interaction of amphotericin B and its selected derivatives with membranes: molecular modeling studies. Chem Rec. 2006;6:320–332. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

