Fine-tuning of neuronal architecture requires two profilin isoforms
- PMID: 20798032
- PMCID: PMC2936622
- DOI: 10.1073/pnas.1004406107
Fine-tuning of neuronal architecture requires two profilin isoforms
Abstract
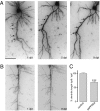
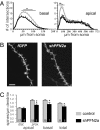
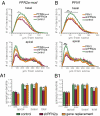
Two profilin isoforms (PFN1 and PFN2a) are expressed in the mammalian brain. Although profilins are essential for regulating actin dynamics in general, the specific role of these isoforms in neurons has remained elusive. We show that knockdown of the neuron-specific PFN2a results in a significant reduction in dendrite complexity and spine numbers of hippocampal neurons. Overexpression of PFN1 in PFN2a-deficient neurons prevents the loss of spines but does not restore dendritic complexity. Furthermore, we show that profilins are involved in differentially regulating actin dynamics downstream of the pan-neurotrophin receptor (p75(NTR)), a receptor engaged in modulating neuronal morphology. Overexpression of PFN2a restores the morphological changes in dendrites caused by p75(NTR) overexpression, whereas PFN1 restores the normal spine density. Our data assign specific functions to the two PFN isoforms, possibly attributable to different affinities for potent effectors also involved in actin dynamics, and suggest that they are important for the signal-dependent fine-tuning of neuronal architecture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Carlsson L, Nyström LE, Sundkvist I, Markey F, Lindberg U. Actin polymerizability is influenced by profilin, a low molecular weight protein in non-muscle cells. J Mol Biol. 1977;115:465–483. - PubMed
-
- Jockusch BM, Murk K, Rothkegel M. The profile of profilins. Rev Physiol Biochem Pharmacol. 2007;159:131–149. - PubMed
-
- Neuhoff H, et al. The actin-binding protein profilin I is localized at synaptic sites in an activity-regulated manner. Eur J Neurosci. 2005;21:15–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

