Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats
- PMID: 20798034
- PMCID: PMC2936617
- DOI: 10.1073/pnas.1010209107
Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats
Abstract
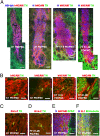
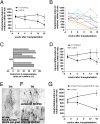
Recent advances in deriving induced pluripotent stem (iPS) cells from patients offer new possibilities for biomedical research and clinical applications, as these cells could be used for autologous transplantation. We differentiated iPS cells from patients with Parkinson's disease (PD) into dopaminergic (DA) neurons and show that these DA neurons can be transplanted without signs of neurodegeneration into the adult rodent striatum. The PD patient iPS (PDiPS) cell-derived DA neurons survived at high numbers, showed arborization, and mediated functional effects in an animal model of PD as determined by reduction of amphetamine- and apomorphine-induced rotational asymmetry, but only a few DA neurons projected into the host striatum at 16 wk after transplantation. We next applied FACS for the neural cell adhesion molecule NCAM on differentiated PDiPS cells before transplantation, which resulted in surviving DA neurons with functional effects on amphetamine-induced rotational asymmetry in a 6-OHDA animal model of PD. Morphologically, we found that PDiPS cell-derived non-DA neurons send axons along white matter tracts into specific close and remote gray matter target areas in the adult brain. Such findings establish the transplantation of human PDiPS cell-derived neurons as a long-term in vivo method to analyze potential disease-related changes in a physiological context. Our data also demonstrate proof of principle of survival and functional effects of PDiPS cell-derived DA neurons in an animal model of PD and encourage further development of differentiation protocols to enhance growth and function of implanted PDiPS cell-derived DA neurons in regard to potential therapeutic applications.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

