Notch signaling specifies prosensory domains via lateral induction in the developing mammalian inner ear
- PMID: 20798046
- PMCID: PMC2936601
- DOI: 10.1073/pnas.1002827107
Notch signaling specifies prosensory domains via lateral induction in the developing mammalian inner ear
Abstract
During inner ear morphogenesis, the process of prosensory specification defines the specific regions of the otic epithelium that will give rise to the six separate inner ear organs essential for hearing and balance. The mechanism of prosensory specification is not fully understood, but there is evidence that the Notch intercellular signaling pathway plays a critical role. The Notch ligand Jagged1 (Jag1) is expressed in the prosensory domains, and mutation of Jag1 impairs sensory formation. Furthermore, pharmacological inhibition of Notch in vitro during prosensory specification disrupts the prosensory process. Additionally, activation of Notch by cDNA electroporation in chick otocysts results in formation of ectopic sensory patches. Here we test whether Notch activity is sufficient for prosensory specification in the mouse, using a Cre-/loxP approach to conditionally activate the Notch pathway in nonsensory regions of the inner ear epithelia during different stages of otic vesicle morphogenesis. We find that broad ectopic activation of Notch at very early developmental stages causes induction of prosensory markers throughout the entire otic epithelium. At later stages of development, activation of Notch in nonsensory regions leads to induction of sensory patches that later differentiate to form complete ectopic sensory structures. Activation of Notch in isolated nonsensory cells results in lateral induction of Jag1 expression in neighboring cells and spreading of prosensory specification to the adjacent cells through an intercellular mechanism. These results support a model where activation of Notch and propagation through lateral induction promote prosensory character in specific regions of the developing otocyst.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
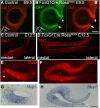
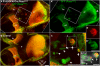
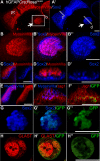
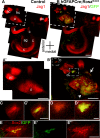
References
-
- Adam J, et al. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: Parallels with Drosophila sense-organ development. Development. 1998;125:4645–4654. - PubMed
-
- Lewis AK, Frantz GD, Carpenter DA, de Sauvage FJ, Gao WQ. Distinct expression patterns of Notch family receptors and ligands during development of the mammalian inner ear. Mech Dev. 1998;78:159–163. - PubMed
-
- Morrison A, Hodgetts C, Gossler A, Hrabé de Angelis M, Lewis J. Expression of Delta1 and Serrate1 (Jagged1) in the mouse inner ear. Mech Dev. 1999;84:169–172. - PubMed
-
- Murata J, Tokunaga A, Okano H, Kubo T. Mapping of Notch activation during cochlear development in mice: Implications for determination of prosensory domain and cell fate diversification. J Comp Neurol. 2006;497:502–518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

