The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila
- PMID: 20798049
- PMCID: PMC2936591
- DOI: 10.1073/pnas.1004060107
The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila
Abstract
Defects in apical-basal cell polarity and abnormal expression of cell polarity determinants are often associated with cancer in vertebrates. In Drosophila, abnormal expression of apical-basal determinants can cause neoplastic phenotypes, including loss of cell polarity and overproliferation. However, the pathways through which apical-basal polarity determinants affect growth are poorly understood. Here, we investigated the mechanism by which the apical determinant Crumbs (Crb) affects growth in Drosophila imaginal discs. Overexpression of Crb causes severe overproliferation, and we found that loss of Crb similarly results in overgrowth of imaginal discs. Crb gain and loss of function caused defects in Hippo signaling, a key signaling pathway that controls tissue growth in Drosophila and mammals. Manipulation of Crb levels caused the up-regulation of Hippo target genes, genetically interacted with known Hippo pathway components, and required Yorkie, a transcriptional coactivator that acts downstream in the Hippo pathway, for target gene induction and overgrowth. Interestingly, Crb regulates growth and cell polarity through different motifs in its intracellular domain. A juxtamembrane FERM domain-binding motif is responsible for growth regulation and induction of Hippo target gene expression, whereas Crb uses a PDZ-binding motif to form a complex with other polarity factors. The Hippo pathway component Expanded, an apically localized adaptor protein, is mislocalized in both crb mutant cells and Crb overexpressing tissues, whereas the other Hippo pathway components, Fat and Merlin, are unaffected. Taken together, our data show that Crb regulates growth through Hippo signaling, and thus identify Crb as a previously undescribed upstream input into the Hippo pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
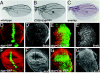

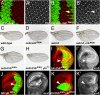
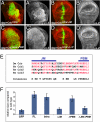

References
-
- Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet. 2001;35:747–784. - PubMed
-
- Dow LE, Humbert PO. Polarity regulators and the control of epithelial architecture, cell migration, and tumorigenesis. Int Rev Cytol. 2007;262:253–302. - PubMed
-
- Assémat E, Bazellières E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778:614–630. - PubMed
-
- Humbert PO, et al. Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene. 2008;27:6888–6907. - PubMed
-
- Hariharan IK, Bilder D. Regulation of imaginal disc growth by tumor-suppressor genes in Drosophila. Annu Rev Genet. 2006;40:335–361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

