Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression
- PMID: 20798195
- PMCID: PMC3228037
- DOI: 10.1634/theoncologist.2010-0129
Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression
Abstract
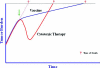
Therapeutic cancer vaccines represent a new class of agents in the treatment of cancer. Sipuleucel-T is an antigen-presenting cell-based vaccine that recently demonstrated a significant 4.8-month improvement in overall survival in advanced prostate cancer patients and was well tolerated. The findings of that study have been met with skepticism, primarily because the agent did not change initial disease progression and yet led to longer survival. Although the commonly accepted treatment paradigm suggests that treatments should initially decrease tumor volume, perhaps vaccines work differently. Vaccines may induce delayed responses not seen in the first few months of therapy or they may initiate a dynamic immune response that ultimately slows the tumor growth rate, resulting in longer survival. Subsequent therapies may also combine with the induced immune response, resulting in a combination that is more effective than conventional treatments alone. Also, other treatments may alter tumor-associated antigen expression, enhancing the immune response. Future trials are currently planned to investigate these hypotheses; however, the results of the sipuleucel-T vaccine in prostate cancer should not be dismissed. Results with another vaccine in prostate cancer are similar, perhaps suggesting a class effect. In a broader context, clinicians may need to reconsider how they measure success. Several agents have been approved that produce superior disease progression results, but do not affect overall survival. Given the toxicity and costs of cancer therapies, perhaps studies should put more weight on long-term survival endpoints than on short-term endpoints that may be less consequential.
Conflict of interest statement
This article discusses experimental vaccines.
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the authors or independent peer reviewers.
Figures
References
-
- Eisenberger M. Discussion: Immunotherapy for Prostate Cancer? Mario A Eisenberger, MD, Discussant. [accessed June 25, 2010]. Available at http://www.asco.org/ASCOv2/MultiMedia/Virtual+Meeting?&vmview=vm_session....
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. - PubMed
-
- Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. - PubMed
-
- James ND, Caty A, Borre M, et al. Safety and efficacy of the specific endothelin-A receptor antagonist ZD4054 in patients with hormone-resistant prostate cancer and bone metastases who were pain free or mildly symptomatic: A double-blind, placebo-controlled, randomised, phase 2 trial. Eur Urol. 2009;55:1112–1123. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

