Deletion of the pH sensor GPR4 decreases renal acid excretion
- PMID: 20798260
- PMCID: PMC3013533
- DOI: 10.1681/ASN.2009050477
Deletion of the pH sensor GPR4 decreases renal acid excretion
Abstract
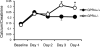
Proton receptors are G protein-coupled receptors that accept protons as ligands and function as pH sensors. One of the proton receptors, GPR4, is relatively abundant in the kidney, but its potential role in acid-base homeostasis is unknown. In this study, we examined the distribution of GPR4 in the kidney, its function in kidney epithelial cells, and the effects of its deletion on acid-base homeostasis. We observed GPR4 expression in the kidney cortex, in the outer and inner medulla, in isolated kidney collecting ducts, and in cultured outer and inner medullary collecting duct cells (mOMCD1 and mIMCD3). Cultured mOMCD1 cells exhibited pH-dependent accumulation of intracellular cAMP, characteristic of GPR4 activation; GPR4 knockdown attenuated this accumulation. In vivo, deletion of GPR4 decreased net acid secretion by the kidney and resulted in a nongap metabolic acidosis, indicating that GPR4 is required to maintain acid-base homeostasis. Collectively, these findings suggest that GPR4 is a pH sensor with an important role in regulating acid secretion in the kidney collecting duct.
Figures

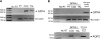

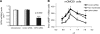


References
-
- Kurtz I, Maher T, Hulter HN, Schambelan M, Sebastian A: Effect of diet on plasma acid-base composition in normal humans. Kidney Int 24: 670–680, 1983 - PubMed
-
- Sun X, Petrovic S: Decreased net acid secretion in pH sensor knockout mice [Abstract]. J Am Soc Nephrol 19: 40A, 2008
-
- Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K: Proton-sensing G-protein-coupled receptors. Nature 425: 93–98, 2003 - PubMed
-
- Sun X, McGraw DW, Petrovic S: The proton-sensing receptor, GPR4, is a good candidate for an acid sensor in the kidney [Abstract]. J Am Soc Nephrol 17: 14A, 2006
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

