Phosphoenolpyruvate provision to plastids is essential for gametophyte and sporophyte development in Arabidopsis thaliana
- PMID: 20798327
- PMCID: PMC2947176
- DOI: 10.1105/tpc.109.073171
Phosphoenolpyruvate provision to plastids is essential for gametophyte and sporophyte development in Arabidopsis thaliana
Abstract
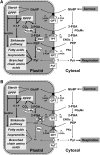
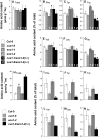
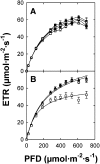
Restriction of phosphoenolpyruvate (PEP) supply to plastids causes lethality of female and male gametophytes in Arabidopsis thaliana defective in both a phosphoenolpyruvate/phosphate translocator (PPT) of the inner envelope membrane and the plastid-localized enolase (ENO1) involved in glycolytic PEP provision. Homozygous double mutants of cue1 (defective in PPT1) and eno1 could not be obtained, and homozygous cue1 heterozygous eno1 mutants [cue1/eno1(+/-)] exhibited retarded vegetative growth, disturbed flower development, and up to 80% seed abortion. The phenotypes of diminished oil in seeds, reduced flavonoids and aromatic amino acids in flowers, compromised lignin biosynthesis in stems, and aberrant exine formation in pollen indicate that cue1/eno1(+/-) disrupts multiple pathways. While diminished fatty acid biosynthesis from PEP via plastidial pyruvate kinase appears to affect seed abortion, a restriction in the shikimate pathway affects formation of sporopollonin in the tapetum and lignin in the stem. Vegetative parts of cue1/eno1(+/-) contained increased free amino acids and jasmonic acid but had normal wax biosynthesis. ENO1 overexpression in cue1 rescued the leaf and root phenotypes, restored photosynthetic capacity, and improved seed yield and oil contents. In chloroplasts, ENO1 might be the only enzyme missing for a complete plastidic glycolysis.
Figures




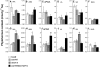

Similar articles
-
Molecular and functional characterization of the plastid-localized Phosphoenolpyruvate enolase (ENO1) from Arabidopsis thaliana.FEBS Lett. 2009 Mar 18;583(6):983-91. doi: 10.1016/j.febslet.2009.02.017. Epub 2009 Feb 15. FEBS Lett. 2009. PMID: 19223001
-
Reticulate leaves and stunted roots are independent phenotypes pointing at opposite roles of the phosphoenolpyruvate/phosphate translocator defective in cue1 in the plastids of both organs.Front Plant Sci. 2014 Apr 8;5:126. doi: 10.3389/fpls.2014.00126. eCollection 2014. Front Plant Sci. 2014. PMID: 24782872 Free PMC article.
-
The phosphoenolpyruvate/phosphate translocator is required for phenolic metabolism, palisade cell development, and plastid-dependent nuclear gene expression.Plant Cell. 1999 Sep;11(9):1609-22. doi: 10.1105/tpc.11.9.1609. Plant Cell. 1999. PMID: 10488230 Free PMC article.
-
The role of transporters in supplying energy to plant plastids.J Exp Bot. 2011 Apr;62(7):2381-92. doi: 10.1093/jxb/erq361. J Exp Bot. 2011. PMID: 21511915 Review.
-
Phosphoenolpyruvate and Related Metabolic Pathways Contribute to the Regulation of Plant Growth and Development.Int J Mol Sci. 2025 Jan 4;26(1):391. doi: 10.3390/ijms26010391. Int J Mol Sci. 2025. PMID: 39796250 Free PMC article. Review.
Cited by
-
The evolution of the plastid phosphate translocator family.Planta. 2019 Jul;250(1):245-261. doi: 10.1007/s00425-019-03161-y. Epub 2019 Apr 16. Planta. 2019. PMID: 30993402
-
Comparative Analysis on the Evolution of Flowering Genes in Sugar Pathway in Brassicaceae.Genes (Basel). 2022 Sep 28;13(10):1749. doi: 10.3390/genes13101749. Genes (Basel). 2022. PMID: 36292634 Free PMC article.
-
Pollen tube growth: where does the energy come from?Plant Signal Behav. 2014;9(12):e977200. doi: 10.4161/15592324.2014.977200. Plant Signal Behav. 2014. PMID: 25482752 Free PMC article. Review.
-
Carbonic Anhydrases Function in Anther Cell Differentiation Downstream of the Receptor-Like Kinase EMS1.Plant Cell. 2017 Jun;29(6):1335-1356. doi: 10.1105/tpc.16.00484. Epub 2017 May 18. Plant Cell. 2017. PMID: 28522549 Free PMC article.
-
Photosynthetic Electron Flows and Networks of Metabolite Trafficking to Sustain Metabolism in Photosynthetic Systems.Plants (Basel). 2024 Oct 28;13(21):3015. doi: 10.3390/plants13213015. Plants (Basel). 2024. PMID: 39519934 Free PMC article. Review.
References
-
- Alexander M.P. (1969). Differential staining of aborted and nonaborted pollen. Stain Technol. 44: 117–122 - PubMed
-
- Ariizumi T., Hatakeyama K., Hinata K., Inatsugi R., Nishida I., Sato S., Kato T., Tabata S., Toriyama K. (2004). Disruption of the novel plant protein NEF1 affects lipid accumulation in plastids of the tapetum and exine formation of pollen, resulting in male sterility in Arabidopsis thaliana. Plant J. 39: 170–181 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

