Local regulation of arterial L-type calcium channels by reactive oxygen species
- PMID: 20798361
- PMCID: PMC2967383
- DOI: 10.1161/CIRCRESAHA.110.217018
Local regulation of arterial L-type calcium channels by reactive oxygen species
Abstract
Rationale: Reactive oxygen species (ROS) are implicated in the development of cardiovascular disease, and oxidants are important signaling molecules in many cell types. Recent evidence suggests that localized subcellular compartmentalization of ROS generation is an important feature of ROS signaling. However, mechanisms that transduce localized subcellular changes in redox status to functionally relevant changes in cellular processes such as Ca(2+) influx are poorly understood.
Objective: To test the hypothesis that ROS regulate L-type Ca(2+) channel activity in cerebral arterial smooth muscle.
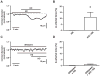
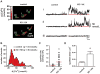
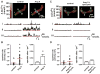
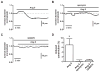
Methods and results: Using a total internal reflection fluorescence imaging-based approach, we found that highly localized subplasmalemmal generation of endogenous ROS preceded and colocalized with sites of enhanced L-type Ca(2+) channel sparklet activity in isolated cerebral arterial smooth muscle cells. Consistent with this observation and our hypothesis, exogenous ROS increased localized L-type Ca(2+) channel sparklet activity in isolated arterial myocytes via activation of protein kinase Cα and when applied to intact cerebral arterial segments, exogenous ROS increased arterial tone in an L-type Ca(2+) channel-dependent fashion. Furthermore, angiotensin II-dependent stimulation of local L-type Ca(2+) channel sparklet activity in isolated cells and contraction of intact arteries was abolished following inhibition of NADPH oxidase.
Conclusions: Our data support a novel model of local oxidative regulation of Ca(2+) influx where vasoconstrictors coupled to NAPDH oxidase (eg, angiotensin II) induce discrete sites of ROS generation resulting in oxidative activation of adjacent protein kinase Cα molecules that in turn promote local sites of enhanced L-type Ca(2+) channel activity, resulting in increased Ca(2+) influx and contraction.
Figures


References
-
- Adiga IK, Nair RR. Multiple signaling pathways coordinately mediate reactive oxygen species dependent cardiomyocyte hypertrophy. Cell Biochem Funct. 2008;26:346–351. - PubMed
-
- Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4:461–470. - PubMed
-
- Hordijk PL. Regulation of NADPH oxidases: The role of Rac proteins. Circ Res. 2006;98:453–462. - PubMed
-
- Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: Clinical implications and therapeutic possibilities. Diabetes Care. 2008;31 (Suppl 2):S170–180. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

