Differential toxicity of DNA adducts of mitomycin C
- PMID: 20798760
- PMCID: PMC2925095
- DOI: 10.4061/2010/698960
Differential toxicity of DNA adducts of mitomycin C
Abstract
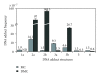
The clinically used antitumor agent mitomycin C (MC) alkylates DNA upon reductive activation, forming six covalent DNA adducts in this process. This paper focuses on differential biological effects of individual adducts in various mammalian cell cultures, observed in the authors' laboratories. Evidence is reviewed that various adducts are capable of inducing different cell death pathways in cancer cells. This evidence is derived from a parallel study of MC and its derivatives 2,7-diaminomitosene (2,7-DAM) which is the main metabolite of MC and forms two monoadducts with DNA, and decarbamoyl mitomycin C (DMC), which alkylates and crosslinks DNA, predominantly with a chirality opposite to that of the DNA adducts of MC. Specifically, 2,7-DAM is not cytotoxic and does not activate the p53 pathway while MC and DMC are cytotoxic and able to activate the p53 pathway. DMC is more cytotoxic than MC and can also kill p53-deficient cells by inducing degradation of Checkpoint 1 protein, which is not seen with MC treatment of the p53-deficient cells. This difference in the cell death pathways activated by the MC and DMC is attributed to differential signaling by the DNA adducts of DMC. We hypothesize that the different chirality of the adduct-to-DNA linkage has a modulating influence on the choice of pathway. Future studies will be directed to elucidate mechanisms of MC- and DMC-DNA adduct signaling in a structure-dependent context.
Figures
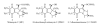


References
-
- Hata T, Hoshi T, Kanamori K, et al. Mitomycin, a new antibiotic from Streptomyces. I. The Journal of Antibiotics . 1956;9:141–146. . - PubMed
-
- Carter SK, Crooke STE. Mitomycin C: Current Status and New Developments . New York, NY, USA: Academic Press; 1979. .
-
- Armstrong RW, Salvati ME, Nguyen M. Novel interstrand cross-links induced by the antitumor antibiotic carzinophilin/azinomycin B. Journal of the American Chemical Society . 1992;114(8):3144–3145. .
-
- Tomasz M, Lipman R, Chowdary D. Isolation and structure of a covalent cross-link adduct between mitomycin C and DNA. Science . 1987;235(4793):1204–1208. . - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

