Molecular and therapeutic potential and toxicity of valproic acid
- PMID: 20798865
- PMCID: PMC2926634
- DOI: 10.1155/2010/479364
Molecular and therapeutic potential and toxicity of valproic acid
Abstract
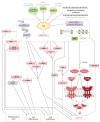
Valproic acid (VPA), a branched short-chain fatty acid, is widely used as an antiepileptic drug and a mood stabilizer. Antiepileptic properties have been attributed to inhibition of Gamma Amino Butyrate (GABA) transaminobutyrate and of ion channels. VPA was recently classified among the Histone Deacetylase Inhibitors, acting directly at the level of gene transcription by inhibiting histone deacetylation and making transcription sites more accessible. VPA is a widely used drug, particularly for children suffering from epilepsy. Due to the increasing number of clinical trials involving VPA, and interesting results obtained, this molecule will be implicated in an increasing number of therapies. However side effects of VPA are substantially described in the literature whereas they are poorly discussed in articles focusing on its therapeutic use. This paper aims to give an overview of the different clinical-trials involving VPA and its side effects encountered during treatment as well as its molecular properties.
Figures
Similar articles
-
Hidden pharmacological activities of valproic acid: A new insight.Biomed Pharmacother. 2021 Oct;142:112021. doi: 10.1016/j.biopha.2021.112021. Epub 2021 Aug 25. Biomed Pharmacother. 2021. PMID: 34463268 Review.
-
Valproic acid selectively suppresses the formation of inhibitory synapses in cultured cortical neurons.Neurosci Lett. 2014 May 21;569:142-7. doi: 10.1016/j.neulet.2014.03.066. Epub 2014 Apr 5. Neurosci Lett. 2014. PMID: 24708928
-
Suppression of adiponectin gene expression by histone deacetylase inhibitor valproic acid.Endocrinology. 2006 Feb;147(2):865-74. doi: 10.1210/en.2005-1030. Epub 2005 Nov 10. Endocrinology. 2006. PMID: 16282359
-
Biochemical, molecular and epigenetic mechanisms of valproic acid neuroprotection.Curr Mol Pharmacol. 2009 Jan;2(1):95-109. doi: 10.2174/1874467210902010095. Curr Mol Pharmacol. 2009. PMID: 20021450 Review.
-
Experimental studies and controlled clinical testing of valproate and vigabatrin.Acta Neurol Scand. 1988 Oct;78(4):241-70. doi: 10.1111/j.1600-0404.1988.tb03655.x. Acta Neurol Scand. 1988. PMID: 3146860 Review.
Cited by
-
Effect of sodium valproate on the toxicity of cyclophosphamide in the testes of mice: influence of pre- and post-treatment schedule.Toxicol Int. 2013 Jan;20(1):68-76. doi: 10.4103/0971-6580.111562. Toxicol Int. 2013. PMID: 23833441 Free PMC article.
-
The Effect of Neuropsychiatric Drugs on the Oxidation-Reduction Balance in Therapy.Int J Mol Sci. 2024 Jul 3;25(13):7304. doi: 10.3390/ijms25137304. Int J Mol Sci. 2024. PMID: 39000411 Free PMC article. Review.
-
Prophylactic valproic acid treatment prevents schizophrenia-related behaviour in Disc1-L100P mutant mice.PLoS One. 2012;7(12):e51562. doi: 10.1371/journal.pone.0051562. Epub 2012 Dec 18. PLoS One. 2012. PMID: 23272119 Free PMC article.
-
Sodium valproate and 5-aza-2'-deoxycytidine differentially modulate DNA demethylation in G1 phase-arrested and proliferative HeLa cells.Sci Rep. 2019 Dec 3;9(1):18236. doi: 10.1038/s41598-019-54848-x. Sci Rep. 2019. PMID: 31796828 Free PMC article.
-
The evolution of knowledge on genes associated with human diseases.iScience. 2021 Dec 11;25(1):103610. doi: 10.1016/j.isci.2021.103610. eCollection 2022 Jan 21. iScience. 2021. PMID: 35005554 Free PMC article.
References
-
- Burton B. On the propyl derivatives and decomposition products of ethylacetoacetate. American Chemical Journal. 1882;3:385–395.
-
- Meunier H, Carraz G, Neunier Y, Eymard P, Aimard M. Pharmacodynamic properties of N-dipropylacetic acid. Thérapie. 1963;18:435–438. - PubMed
-
- Lebreton S, Carraz G, Behriel H, Meunier H. Pharmacodynamic properties of 2,2-dipropylacetic Acid. III. Therapie. 1984;19:457–467. - PubMed
-
- Lebreton S, Carraz G, Meunier H, Behriel H. Pharmacodynamic properties of 2,2-dipropylacetic Acid. 2d report on its anti-epileptic properties. Therapie. 1964;19:451–456. - PubMed
-
- Mesdjian E, Ciesielski L, Valli M. Sodium valproate: kinetic profile and effects on GABA levels in various brain areas of the rat. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1982;6(3):223–233. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
