Exploration of the interrupted Fischer indolization reaction
- PMID: 20798890
- PMCID: PMC2925302
- DOI: 10.1016/j.tet.2010.02.050
Exploration of the interrupted Fischer indolization reaction
Abstract
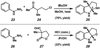
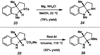
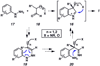
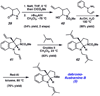
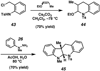
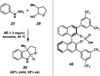
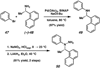
A convergent method to access the fused indoline ring system present in a multitude of bioactive molecules has been developed. The strategy involves the condensation of hydrazines with latent aldehydes to ultimately deliver indoline-containing products by way of an interrupted Fischer indolization sequence. The method is convergent, mild, operationally simple, broad in scope, and can be used to access enantioenriched products. In addition, our approach is amenable to the synthesis of furoindoline and pyrrolidinoindoline natural products as demonstrated by the concise formal total syntheses of physovenine and debromoflustramine B. The strategy will likely enable the synthesis of more complex targets such as the communesin alkaloids.
Figures


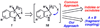
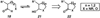
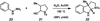

Similar articles
-
An interrupted Fischer indolization approach toward fused indoline-containing natural products.Org Lett. 2009 Aug 6;11(15):3458-61. doi: 10.1021/ol901383j. Org Lett. 2009. PMID: 19601608
-
Synthesis of Fused Indolines by Interrupted Fischer Indolization in a Microfluidic Reactor.Tetrahedron Lett. 2019 Jan 17;60(3):322-326. doi: 10.1016/j.tetlet.2018.12.045. Epub 2018 Dec 19. Tetrahedron Lett. 2019. PMID: 30631216 Free PMC article.
-
Interrupted Fischer indolization approach toward the communesin alkaloids and perophoramidine.Org Lett. 2012 Sep 7;14(17):4556-9. doi: 10.1021/ol302023q. Epub 2012 Aug 20. Org Lett. 2012. PMID: 22905746 Free PMC article.
-
Diversity-Oriented Synthetic Approaches for Furoindoline: A Review.Curr Org Synth. 2019;16(3):342-368. doi: 10.2174/1570179416666190328211509. Curr Org Synth. 2019. PMID: 31984898 Review.
-
Recent Advances on the Total Syntheses of Communesin Alkaloids and Perophoramidine.Chemistry. 2015 Nov 9;21(46):16318-43. doi: 10.1002/chem.201501735. Epub 2015 Sep 10. Chemistry. 2015. PMID: 26353936 Free PMC article. Review.
Cited by
-
Synthesis of indole derivatives as prevalent moieties present in selected alkaloids.RSC Adv. 2021 Oct 15;11(53):33540-33612. doi: 10.1039/d1ra05972f. eCollection 2021 Oct 8. RSC Adv. 2021. PMID: 35497516 Free PMC article. Review.
-
Enantioselective Total Syntheses of Akuammiline Alkaloids (+)-Strictamine, (-)-2(S)-Cathafoline, and (-)-Aspidophylline A.J Am Chem Soc. 2016 Feb 3;138(4):1162-5. doi: 10.1021/jacs.5b12880. Epub 2016 Jan 19. J Am Chem Soc. 2016. PMID: 26783944 Free PMC article.
-
Why do some Fischer indolizations fail?J Am Chem Soc. 2011 Apr 20;133(15):5752-5. doi: 10.1021/ja201035b. Epub 2011 Mar 28. J Am Chem Soc. 2011. PMID: 21443189 Free PMC article.
-
Total synthesis of (+)-gliocladin C enabled by visible-light photoredox catalysis.Angew Chem Int Ed Engl. 2011 Oct 4;50(41):9655-9. doi: 10.1002/anie.201103145. Epub 2011 Jul 12. Angew Chem Int Ed Engl. 2011. PMID: 21751318 Free PMC article. No abstract available.
-
Enantioselective Total Syntheses of Methanoquinolizidine-Containing Akuammiline Alkaloids and Related Studies.J Am Chem Soc. 2018 May 23;140(20):6483-6492. doi: 10.1021/jacs.8b03404. Epub 2018 May 15. J Am Chem Soc. 2018. PMID: 29694031 Free PMC article.
References
-
- Hudlicky T, Reed JW. The Way of Synthesis. Weinheim: Wiley–VCH; 2007.
- Wender PA, Verman VA, Paxton TJ, Pillow TH. Acc. Chem. Res. 2008;41:40–49. - PubMed
-
-
For a review of these alkaloids, see: Takano S, Ogasawara K. Alkaloids. 1989;36:225–251.
-
-
-
Physostigmine (3) has been used to treat glaucoma, Alzheimer’s disease, and myasthenia gravis. For a pertinent review, see Triggle DJ, Mitchell JM, Filler R. CNS Drug Rev. 1998;4:87–136.
-
-
- Carle JS, Christophersen C. J. Am. Chem. Soc. 1979;101:4012–4013.
- Holst PB, Anthoni U, Christophersen C, Nielsen PH. J. Nat. Prod. 1994;57:997–1000. - PubMed
-
-
For a pertinent review, see: Steven A, Overman LE. Angew. Chem. Int. Ed. 2007;46:5488–5508.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
