Excitation-wavelength dependent fluorescence of ethyl 5-(4-aminophenyl)-3-amino-2,4-dicyanobenzoate
- PMID: 20798980
- PMCID: PMC3032210
- DOI: 10.1007/s10895-010-0711-4
Excitation-wavelength dependent fluorescence of ethyl 5-(4-aminophenyl)-3-amino-2,4-dicyanobenzoate
Abstract
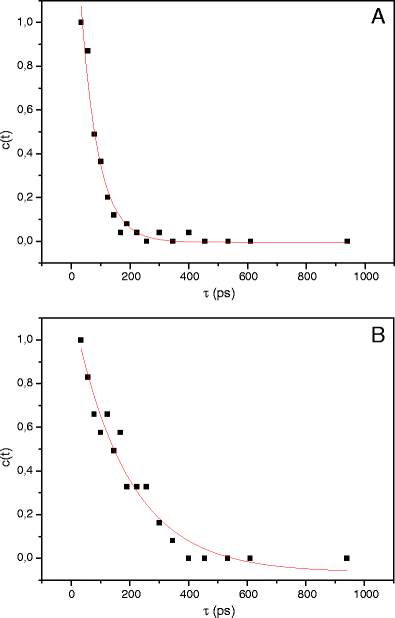
The excitation wavelength dependence of the steady-state and time-resolved emission spectra of ethyl 5-(4-aminophenyl)-3-amino-2,4-dicyanobenzoate (EAADCy) in tetrahydrofuran (THF) at room temperature has been examined. It is found that the ratio of the fluorescence intensity of the long-wavelength and short-wavelength fluorescence bands strongly depends on the excitation wavelength, whereas the wavelengths of the fluorescence excitation and fluorescence bands maxima are independent on the observation/excitation wavelengths. The dynamic Stokes shift of fluorophore in locally excited (LE) and intramolecular charge transfer (ICT) states has been studied with a time resolution about 30 ps. The difference between Stokes shift in the LE and ICT states was attributed to the solvent response to the large photoinduced dipole moment of EAADCy in the fluorescent charge transfer state. On this base we can state that, the relaxation of the polar solvent molecules around the fluorophore was observed.
Figures

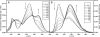



References
-
- Lakowicz JR. Principle of fluorescence spectroscopy. New York: Kluwer Academic/Plenum Publishing Corporation; 2006.
-
- Demchenko AP. Luminescence. 2002;17:19. - PubMed
-
- Sengupta B, Guharay J, Chakraborty A, Sengupta PK. Spectrochim Acta A. 2002;58:2005. - PubMed
-
- Kallir AJ, Suter GW, Wild UP. J. Phys. Chem. 1987;91:60.
LinkOut - more resources
Full Text Sources
Other Literature Sources

