ETV5 regulates sertoli cell chemokines involved in mouse stem/progenitor spermatogonia maintenance
- PMID: 20799334
- PMCID: PMC3109872
- DOI: 10.1002/stem.508
ETV5 regulates sertoli cell chemokines involved in mouse stem/progenitor spermatogonia maintenance
Abstract
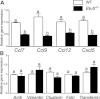
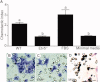
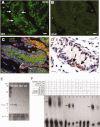
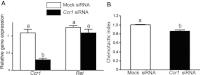
Spermatogonial stem cells are the only stem cells in the body that transmit genetic information to offspring. Although growth factors responsible for self-renewal of these cells are known, the factors and mechanisms that attract and physically maintain these cells within their microenvironment are poorly understood. Mice with targeted disruption of Ets variant gene 5 (Etv5) show total loss of stem/progenitor spermatogonia following the first wave of spermatogenesis, resulting in a Sertoli cell-only phenotype and aspermia. Microarray analysis of primary Sertoli cells from Etv5 knockout (Etv5(-/-)) versus wild-type (WT) mice revealed significant decreases in expression of several chemokines. Chemotaxis assays demonstrated that migration of stem/progenitor spermatogonia toward Etv5(-/-) Sertoli cells was significantly decreased compared to migration toward WT Sertoli cells. Interestingly, differentiating spermatogonia, spermatocytes, and round spermatids were not chemoattracted by WT Sertoli cells, whereas stem/progenitor spermatogonia showed a high and significant chemotactic index. Rescue assays using recombinant chemokines indicated that C-C-motif ligand 9 (CCL9) facilitates Sertoli cell chemoattraction of stem/progenitor spermatogonia, which express C-C-receptor type 1 (CCR1). In addition, there is protein-DNA interaction between ETV5 and Ccl9, suggesting that ETV5 might be a direct regulator of Ccl9 expression. Taken together, our data show for the first time that Sertoli cells are chemoattractive for stem/progenitor spermatogonia, and that production of specific chemokines is regulated by ETV5. Therefore, changes in chemokine production and consequent decreases in chemoattraction by Etv5(-/-) Sertoli cells helps to explain stem/progenitor spermatogonia loss in Etv5(-/-) mice.
Figures

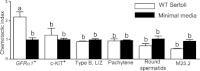

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

