Functional hot spots in human ATP-binding cassette transporter nucleotide binding domains
- PMID: 20799350
- PMCID: PMC3005782
- DOI: 10.1002/pro.491
Functional hot spots in human ATP-binding cassette transporter nucleotide binding domains
Abstract
The human ATP-binding cassette (ABC) transporter superfamily consists of 48 integral membrane proteins that couple the action of ATP binding and hydrolysis to the transport of diverse substrates across cellular membranes. Defects in 18 transporters have been implicated in human disease. In hundreds of cases, disease phenotypes and defects in function can be traced to nonsynonymous single nucleotide polymorphisms (nsSNPs). The functional impact of the majority of ABC transporter nsSNPs has yet to be experimentally characterized. Here, we combine experimental mutational studies with sequence and structural analysis to describe the impact of nsSNPs in human ABC transporters. First, the disease associations of 39 nsSNPs in 10 transporters were rationalized by identifying two conserved loops and a small α-helical region that may be involved in interdomain communication necessary for transport of substrates. Second, an approach to discriminate between disease-associated and neutral nsSNPs was developed and tailored to this superfamily. Finally, the functional impact of 40 unannotated nsSNPs in seven ABC transporters identified in 247 ethnically diverse individuals studied by the Pharmacogenetics of Membrane Transporters consortium was predicted. Three predictions were experimentally tested using human embryonic kidney epithelial (HEK) 293 cells stably transfected with the reference multidrug resistance transporter 4 and its variants to examine functional differences in transport of the antiviral drug, tenofovir. The experimental results confirmed two predictions. Our analysis provides a structural and evolutionary framework for rationalizing and predicting the functional effects of nsSNPs in this clinically important membrane transporter superfamily.
Figures


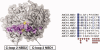

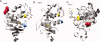
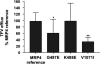
Comment in
-
Predictable difficulty or difficulty to predict.Protein Sci. 2011 Jan;20(1):1-3; author reply 4-5. doi: 10.1002/pro.552. Protein Sci. 2011. PMID: 21082744 Free PMC article. No abstract available.
Similar articles
-
Non-neutral nonsynonymous single nucleotide polymorphisms in human ABC transporters: the first comparison of six prediction methods.Pharmacol Rep. 2011;63(4):924-34. doi: 10.1016/s1734-1140(11)70608-9. Pharmacol Rep. 2011. PMID: 22001980
-
Interactions of tenofovir and tenofovir disoproxil fumarate with drug efflux transporters ABCB1, ABCG2, and ABCC2; role in transport across the placenta.AIDS. 2014 Jan 2;28(1):9-17. doi: 10.1097/QAD.0000000000000112. AIDS. 2014. PMID: 24413260
-
Phenotype prediction of non-synonymous single-nucleotide polymorphisms in human ATP-binding cassette transporter genes.Basic Clin Pharmacol Toxicol. 2011 Feb;108(2):94-114. doi: 10.1111/j.1742-7843.2010.00627.x. Epub 2010 Sep 6. Basic Clin Pharmacol Toxicol. 2011. PMID: 20849526
-
ABC transporters: the power to change.Nat Rev Mol Cell Biol. 2009 Mar;10(3):218-27. doi: 10.1038/nrm2646. Nat Rev Mol Cell Biol. 2009. PMID: 19234479 Free PMC article. Review.
-
The A-loop, a novel conserved aromatic acid subdomain upstream of the Walker A motif in ABC transporters, is critical for ATP binding.FEBS Lett. 2006 Feb 13;580(4):1049-55. doi: 10.1016/j.febslet.2005.12.051. Epub 2005 Dec 22. FEBS Lett. 2006. PMID: 16412422 Review.
Cited by
-
Predictable difficulty or difficulty to predict.Protein Sci. 2011 Jan;20(1):1-3; author reply 4-5. doi: 10.1002/pro.552. Protein Sci. 2011. PMID: 21082744 Free PMC article. No abstract available.
-
Interolog interfaces in protein-protein docking.Proteins. 2015 Nov;83(11):1940-6. doi: 10.1002/prot.24788. Epub 2015 Sep 29. Proteins. 2015. PMID: 25740680 Free PMC article.
-
Mutation spectrum in the ABCC6 gene and genotype-phenotype correlations in a French cohort with pseudoxanthoma elasticum.Genet Med. 2017 Aug;19(8):909-917. doi: 10.1038/gim.2016.213. Epub 2017 Jan 19. Genet Med. 2017. PMID: 28102862
-
ABCC4 is regulated by microRNA-124a and microRNA-506.Biochem Pharmacol. 2014 Feb 1;87(3):515-22. doi: 10.1016/j.bcp.2013.10.017. Epub 2013 Oct 30. Biochem Pharmacol. 2014. PMID: 24184504 Free PMC article.
-
Deleterious nonsynonymous single nucleotide polymorphisms in human solute carriers: the first comparison of three prediction methods.Eur J Drug Metab Pharmacokinet. 2013 Mar;38(1):53-62. doi: 10.1007/s13318-012-0095-8. Epub 2012 May 4. Eur J Drug Metab Pharmacokinet. 2013. PMID: 22555822
References
-
- Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res. 2001;42:1007–1017. - PubMed
-
- Locher K, Lee A, Rees D. The E. coli BtuCD structure: a framework for ABC transporter architecture and mechanism. Science. 2002;296:1091–1098. - PubMed
-
- Dean M, Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu Rev Genomics Hum Genet. 2005;6:123–142. - PubMed
-
- Dawson RJ, Locher KP. Structure of the multidrug ABC transporter Sav1866 from Staphylococcus aureus in complex with AMP-PNP. FEBS Lett. 2007;581:935–938. - PubMed
-
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The protein data bank. Acta Crystallogr D Biol Crystallogr. 2002;58:899–907. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

