Synthesis of diamidopyrrolyl molybdenum complexes relevant to reduction of dinitrogen to ammonia
- PMID: 20799738
- PMCID: PMC2931342
- DOI: 10.1021/ic100856n
Synthesis of diamidopyrrolyl molybdenum complexes relevant to reduction of dinitrogen to ammonia
Abstract
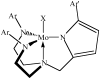
A potentially useful trianionic ligand for the reduction of dinitrogen catalytically by molybdenum complexes is one in which one of the arms in a [(RNCH(2)CH(2))(3)N](3-) ligand is replaced by a 2-mesitylpyrrolyl-alpha-methyl arm, that is, [(RNCH(2)CH(2))(2)NCH(2)(2-MesitylPyrrolyl)](3-) (R = C(6)F(5), 3,5-Me(2)C(6)H(3), or 3,5-t-Bu(2)C(6)H(3)). Compounds have been prepared that contain the ligand in which R = C(6)F(5) ([C(6)F(5)N)(2)Pyr](3-)); they include [(C(6)F(5)N)(2)Pyr]Mo(NMe(2)), [(C(6)F(5)N)(2)Pyr]MoCl, [(C(6)F(5)N)(2)Pyr]MoOTf, and [(C(6)F(5)N)(2)Pyr]MoN. Compounds that contain the ligand in which R = 3,5-t-Bu(2)C(6)H(3) ([Ar(t-Bu)N)(2)Pyr](3-)) include {[(Ar(t-Bu)N)(2)Pyr]Mo(N(2))}Na(15-crown-5), {[(Ar(t-Bu)N)(2)Pyr]Mo(N(2))}[NBu(4)], [(Ar(t-Bu)N)(2)Pyr]Mo(N(2)) (nu(NN) = 2012 cm(-1) in C(6)D(6)), {[(Ar(t-Bu)N)(2)Pyr]Mo(NH(3))}BPh(4), and [(Ar(t-Bu)N)(2)Pyr]Mo(CO). X-ray studies are reported for [(C(6)F(5)N)(2)Pyr]Mo(NMe(2)), [(C(6)F(5)N)(2)Pyr]MoCl, and [(Ar(t-Bu)N)(2)Pyr]MoN. The [(Ar(t-Bu)N)(2)Pyr]Mo(N(2))(0/-) reversible couple is found at -1.96 V (in PhF versus Cp(2)Fe(+/0)), but the [(Ar(t-Bu)N)(2)Pyr]Mo(N(2))(+/0) couple is irreversible. Reduction of {[(Ar(t-Bu)N)(2)Pyr]Mo(NH(3))}BPh(4) under Ar at approximately -1.68 V at a scan rate of 900 mV/s is not reversible. Ammonia in [(Ar(t-Bu)N)(2)Pyr]Mo(NH(3)) can be substituted for dinitrogen in about 2 h if 10 equiv of BPh(3) are present to trap the ammonia that is released. [(Ar(t-Bu)N)(2)Pyr]Mo-N=NH is a key intermediate in the proposed catalytic reduction of dinitrogen that could not be prepared. Dinitrogen exchange studies in [(Ar(t-Bu)N)(2)Pyr]Mo(N(2)) suggest that steric hindrance by the ligand may be insufficient to protect decomposition of [(Ar(t-Bu)N)(2)Pyr]Mo-N=NH through a variety of pathways. Three attempts to reduce dinitrogen catalytically with [(Ar(t-Bu)N)(2)Pyr]Mo(N) as a "catalyst" yielded an average of 1.02 +/- 0.12 equiv of NH(3).
Figures

Similar articles
-
Synthesis of [(DPPNCH2CH2)3N]3- molybdenum complexes (DPP = 3,5-(2,5-Diisopropylpyrrolyl)2C6H3) and studies relevant to catalytic reduction of dinitrogen.J Am Chem Soc. 2010 Jun 23;132(24):8349-58. doi: 10.1021/ja1008213. J Am Chem Soc. 2010. PMID: 20499910 Free PMC article.
-
Molybdenum triamidoamine complexes that contain hexa-tert-butylterphenyl, hexamethylterphenyl, or p-bromohexaisopropylterphenyl substituents. An examination of some catalyst variations for the catalytic reduction of dinitrogen.J Am Chem Soc. 2004 May 19;126(19):6150-63. doi: 10.1021/ja0306415. J Am Chem Soc. 2004. PMID: 15137780
-
Studies relevant to catalytic reduction of dinitrogen to ammonia by molybdenum triamidoamine complexes.Inorg Chem. 2005 Feb 21;44(4):1103-17. doi: 10.1021/ic040095w. Inorg Chem. 2005. PMID: 15859292
-
Catalytic reduction of dinitrogen to ammonia at well-defined single metal sites.Philos Trans A Math Phys Eng Sci. 2005 Apr 15;363(1829):959-69; discussion 1035-40. doi: 10.1098/rsta.2004.1541. Philos Trans A Math Phys Eng Sci. 2005. PMID: 15901545 Review.
-
Cleaving the n,n triple bond: the transformation of dinitrogen to ammonia by nitrogenases.Met Ions Life Sci. 2014;14:147-76. doi: 10.1007/978-94-017-9269-1_7. Met Ions Life Sci. 2014. PMID: 25416394 Review.
Cited by
-
Theoretical studies of homogeneous catalysts mimicking nitrogenase.Molecules. 2011 Jan 10;16(1):442-65. doi: 10.3390/molecules16010442. Molecules. 2011. PMID: 21221062 Free PMC article. Review.
-
Ammonia from dinitrogen at ambient conditions by organometallic catalysts.RSC Adv. 2022 Nov 23;12(52):33567-33583. doi: 10.1039/d2ra06156b. eCollection 2022 Nov 22. RSC Adv. 2022. PMID: 36505716 Free PMC article. Review.
-
Catalytic N2-to-NH3 (or -N2H4) Conversion by Well-Defined Molecular Coordination Complexes.Chem Rev. 2020 Jun 24;120(12):5582-5636. doi: 10.1021/acs.chemrev.9b00638. Epub 2020 Apr 30. Chem Rev. 2020. PMID: 32352271 Free PMC article. Review.
References
-
- Burgess BK. Chem Rev. 1990;90:1377.
- Burgess BK, Lowe DJ. Chem Rev. 1996;96:2983. - PubMed
- Rees DC, Howard JB. Curr Opin Chem Biol. 2000;4:559. - PubMed
- Rees DC, Chan MK, Kim J. Adv Inorg Chem. 1996;40:89.
- Eady RR. Chem Rev. 1996;96:3013. - PubMed
- Howard JB, Rees DC. Chem Rev. 1996;96:2965. - PubMed
- Kim J, Woo D, Rees DC. Biochemistry. 1993;32:7104. - PubMed
- Kim J, Rees DC. Nature. 1992;360:553. - PubMed
- Bolin JT, Ronco AE, Morgan TV, Mortenson LE, Xuong LE. Proc Nat Acad Sci. 1993;90:1078. - PMC - PubMed
- Chen J, Christiansen J, Campobasso N, Bolin JT, Tittsworth RC, Hales BJ, Rehr JJ, Cramer SP. Angew Chem, Int Ed Engl. 1993;32:1592.
- Einsle O, Tezcan FA, Andrade SLA, Schmid B, Yoshida M, Howard JB, Rees DC. Science. 2002;297:1696. - PubMed
- Hardy RWF, Bottomley F, Burns RC. A Treatise on Dinitrogen Fixation. Wiley-Interscience; New York: 1979.
- Christiansen J, Dean DR, Seefeldt LC. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:269. - PubMed
- Dos Santos PC, Igarashi RY, Lee H, Hoffman BM, Seefeldt LC, Dean DR. Acc Chem Res. 2005;38:208. - PubMed
- Dance I. Chem Asian J. 2007;2:936. - PubMed
- Kästner J, Blöchl PE. J Am Chem Soc. 2007;129:2998. - PubMed
-
- Allen AD, Senoff CV. J Chem Soc, Chem Commun. 1965:621.
-
- Fryzuk MD, Johnson SA. Coord Chem Rev. 2000;200–202:379.
- Hidai M, Mizobe Y. Chem Rev. 1995;95:1115.
- Hidai M. Coord Chem Rev. 1999;185–186:99.
- Chatt J, Dilworth JR, Richards RL. Chem Rev. 1978;78:589.
- Richards RL. Coord Chem Rev. 1996;154:83.
- Richards RL. Pure Appl Chem. 1996;68:1521.
- Bazhenova TA, Shilov AE. Coord Chem Rev. 1995;144:69.
- Shilov AE. Russ Chem Bull Int Ed. 2003;2:2555.
- MacKay BA, Fryzuk MD. Chem Rev. 2004;104:385. - PubMed
- Gambarotta S, Scott J. Angew Chem Int Ed. 2004;43:5298. - PubMed
- Hidai M, Mizobe Y. Can J Chem. 2005;83:358.
-
- Knobloch DJ, Lobkovsky E, Chirik PJ. Nat Chem. 2010;2:30. - PubMed
- Hanna TE, Lobkovsky E, Chirik PJ. Organometallics. 2009;28:4079.
- Munha RF, Veiros LF, Duarte MT, Fryzuk MD, Martins AM. Dalton Trans. 2009:7494. - PubMed
- Kozak CM, Mountford P. Angew Chem Int Ed. 2004;43:1186. - PubMed
- Fryzuk MD. Acc Chem Res. 2009;42:127. - PubMed
- Chirik PJ. Dalton Trans. 2007;16 - PubMed
-
- Smith JM, Lachicotte RJ, Holland PL. J Am Chem Soc. 2003;125:15752. - PubMed
- Eckert NA, Smith JM, Lachicotte RJ, Holland PL. Inorg Chem. 2004;43:3306. - PubMed
- Holland PL. Canad J Chem. 2005;83:296.
- Smith JM, Sadique AR, Cundari TR, Rodgers KR, Lukat-Rodgers G, Lachicotte RJ, Flaschenriem CJ, Vela J, Holland PL. J Am Chem Soc. 2006;128:756. - PubMed
- Field LD, Li HL, Magill AM. Inorg Chem. 2009;48:5. - PubMed
- Whited MT, Mankad NP, Lee Y, Oblad PF, Peters JC. Inorg Chem. 2009;48:2507. - PubMed
- Lee Y, Mankad NP, Peters JC. Nat Chem. 2010;2:558. - PMC - PubMed
- Crossland JL, Balesdent CG, Tyler DR. Dalton Trans. 2009:4420. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

