Lifetime-based tomographic multiplexing
- PMID: 20799813
- PMCID: PMC2929260
- DOI: 10.1117/1.3469797
Lifetime-based tomographic multiplexing
Abstract
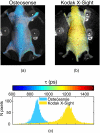
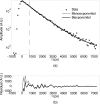
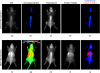
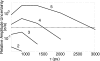
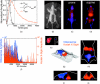
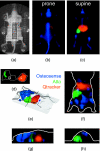
Near-infrared (NIR) fluorescence tomography of multiple fluorophores has previously been limited by the bandwidth of the NIR spectral regime and the broad emission spectra of most NIR fluorophores. We describe in vivo tomography of three spectrally overlapping fluorophores using fluorescence lifetime-based separation. Time-domain images are acquired using a voltage-gated, intensified charge-coupled device (CCD) in free-space transmission geometry with 750 nm Ti:sapphire laser excitation. Lifetime components are fit from the asymptotic portion of fluorescence decay curve and reconstructed separately with a lifetime-adjusted forward model. We use this system to test the in vivo lifetime multiplexing suitability of commercially available fluorophores, and demonstrate lifetime multiplexing in solution mixtures and in nude mice. All of the fluorophores tested exhibit nearly monoexponential decays, with narrow in vivo lifetime distributions suitable for lifetime multiplexing. Quantitative separation of two fluorophores with lifetimes of 1.1 and 1.37 ns is demonstrated for relative concentrations of 1:5. Finally, we demonstrate tomographic imaging of two and three fluorophores in nude mice with fluorophores that localize to distinct organ systems. This technique should be widely applicable to imaging multiple NIR fluorophores in 3-D.
Figures
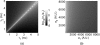


References
-
- Bacskai B. J., Kajdasz S. T., Christie R. H., Carter C., Games D., Seubert P., Schenk D., and Hyman B. T., “Imaging of amyloid-beta deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy,” Nat. Med. ZZZZZZ 7, 369–372 (2001).10.1038/85525 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

