Scanning elastic scattering spectroscopy detects metastatic breast cancer in sentinel lymph nodes
- PMID: 20799832
- PMCID: PMC2917446
- DOI: 10.1117/1.3463005
Scanning elastic scattering spectroscopy detects metastatic breast cancer in sentinel lymph nodes
Abstract
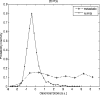
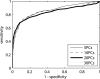
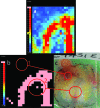
A novel method for rapidly detecting metastatic breast cancer within excised sentinel lymph node(s) of the axilla is presented. Elastic scattering spectroscopy (ESS) is a point-contact technique that collects broadband optical spectra sensitive to absorption and scattering within the tissue. A statistical discrimination algorithm was generated from a training set of nearly 3000 clinical spectra and used to test clinical spectra collected from an independent set of nodes. Freshly excised nodes were bivalved and mounted under a fiber-optic plate. Stepper motors raster-scanned a fiber-optic probe over the plate to interrogate the node's cut surface, creating a 20x20 grid of spectra. These spectra were analyzed to create a map of cancer risk across the node surface. Rules were developed to convert these maps to a prediction for the presence of cancer in the node. Using these analyses, a leave-one-out cross-validation to optimize discrimination parameters on 128 scanned nodes gave a sensitivity of 69% for detection of clinically relevant metastases (71% for macrometastases) and a specificity of 96%, comparable to literature results for touch imprint cytology, a standard technique for intraoperative diagnosis. ESS has the advantage of not requiring a pathologist to review the tissue sample.
Figures



Similar articles
-
Elastic scattering spectroscopy for intraoperative determination of sentinel lymph node status in the breast.J Biomed Opt. 2004 Nov-Dec;9(6):1122-8. doi: 10.1117/1.1802191. J Biomed Opt. 2004. PMID: 15568931 Clinical Trial.
-
Optical scanning for rapid intraoperative diagnosis of sentinel node metastases in breast cancer.Br J Surg. 2010 Aug;97(8):1232-9. doi: 10.1002/bjs.7095. Br J Surg. 2010. PMID: 20593429
-
Elastic scattering spectroscopy for early detection of breast cancer: partially supervised Bayesian image classification of scanned sentinel lymph nodes.J Biomed Opt. 2018 Aug;23(8):1-9. doi: 10.1117/1.JBO.23.8.085004. J Biomed Opt. 2018. PMID: 30132305 Free PMC article.
-
Sentinel lymph node mapping in breast cancer: a critical reappraisal of the internal mammary chain issue.Q J Nucl Med Mol Imaging. 2014 Jun;58(2):114-26. Q J Nucl Med Mol Imaging. 2014. PMID: 24835288 Review.
-
Axillary sentinel lymph node biopsy: an overview.Int J Surg. 2008;6 Suppl 1:S109-12. doi: 10.1016/j.ijsu.2008.12.008. Epub 2008 Dec 13. Int J Surg. 2008. PMID: 19131285 Review.
Cited by
-
Application of Endobronchial Ultrasonography for the Preoperative Detecting Recurrent Laryngeal Nerve Lymph Node Metastasis of Esophageal Cancer.PLoS One. 2015 Sep 15;10(9):e0137400. doi: 10.1371/journal.pone.0137400. eCollection 2015. PLoS One. 2015. PMID: 26372339 Free PMC article.
-
Automated classification of breast pathology using local measures of broadband reflectance.J Biomed Opt. 2010 Nov-Dec;15(6):066019. doi: 10.1117/1.3516594. J Biomed Opt. 2010. PMID: 21198193 Free PMC article.
-
Optical Imaging in Human Lymph Node Specimens for Detecting Breast Cancer Metastases: A Review.Cancers (Basel). 2023 Nov 16;15(22):5438. doi: 10.3390/cancers15225438. Cancers (Basel). 2023. PMID: 38001697 Free PMC article. Review.
-
Towards minimally-invasive, quantitative assessment of chronic kidney disease using optical spectroscopy.Sci Rep. 2019 May 9;9(1):7168. doi: 10.1038/s41598-019-43684-8. Sci Rep. 2019. PMID: 31073168 Free PMC article.
-
Scanning in situ spectroscopy platform for imaging surgical breast tissue specimens.Opt Express. 2013 Jan 28;21(2):2185-94. doi: 10.1364/OE.21.002185. Opt Express. 2013. PMID: 23389199 Free PMC article.
References
-
- Veronesi U., Paganelli G., Viale G., Luini A., Zurrida S., Galimberti V., Intra M., Veronesi P., Robertson C., Maisonneuve P., Renne G., Cicco C. D., Lucia F. D., and Gennari R., “A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer,” N. Engl. J. Med. NEJMAG 349, 546–553 (2003).10.1056/NEJMoa012782 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

