MicroRNA-1 regulates smooth muscle cell differentiation by repressing Kruppel-like factor 4
- PMID: 20799856
- PMCID: PMC3128754
- DOI: 10.1089/scd.2010.0283
MicroRNA-1 regulates smooth muscle cell differentiation by repressing Kruppel-like factor 4
Abstract
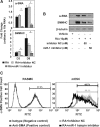
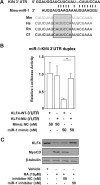
The role of microRNA-1 (miR-1) has been studied in cardiac and skeletal muscle differentiation. However, it remains unexplored in vascular smooth muscle cells (SMCs) differentiation. The aim of this study was to uncover novel targets of and shed light on the function of miR-1 in the context of embryonic stem cell (ESC) differentiation of SMCs in vitro. miR-1 expression is steadily increased during differentiation of mouse ESC to SMCs. Loss-of-function approaches using miR-1 inhibitors uncovered that miR-1 is required for SMC lineage differentiation in ESC-derived SMC cultures, as evidenced by downregulation of SMC-specific markers and decrease of derived SMC population. In addition, bioinformatics analysis unveiled a miR-1 binding site on the Kruppel-like factor 4 (KLF4) 3' untranslated region (3'UTR), in a region that is highly conserved across species. Consistently, miR-1 mimic reduced KLF4 3'UTR luciferase activity, which can be rescued by mutating the miR-1 binding site on the KLF4 3'UTR in the reporter construct. Additionally, repression of the miR-1 expression by miR-1 inhibitor can reverse KLF4 downregulation during ESC-SMC differentiation, which subsequently inhibits SMC differentiation. We conclude that miR-1 plays a critical role in the determination of SMC fate during retinoid acid-induced ESC/SMC differentiation, which may indicate that miR-1 has a role to promote SMC differentiation.
Figures
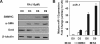
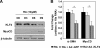
Similar articles
-
The role of microRNA-145 in human embryonic stem cell differentiation into vascular cells.Atherosclerosis. 2011 Dec;219(2):468-74. doi: 10.1016/j.atherosclerosis.2011.09.004. Epub 2011 Sep 9. Atherosclerosis. 2011. PMID: 21945499
-
MicroRNA-22 regulates smooth muscle cell differentiation from stem cells by targeting methyl CpG-binding protein 2.Arterioscler Thromb Vasc Biol. 2015 Apr;35(4):918-29. doi: 10.1161/ATVBAHA.114.305212. Epub 2015 Feb 26. Arterioscler Thromb Vasc Biol. 2015. PMID: 25722434
-
MicroRNA-137 represses Klf4 and Tbx3 during differentiation of mouse embryonic stem cells.Stem Cell Res. 2013 Nov;11(3):1299-313. doi: 10.1016/j.scr.2013.09.001. Epub 2013 Sep 13. Stem Cell Res. 2013. PMID: 24084696
-
Vascular smooth muscle cell phenotypic plasticity: focus on chromatin remodelling.Cardiovasc Res. 2012 Jul 15;95(2):147-55. doi: 10.1093/cvr/cvs098. Epub 2012 Feb 22. Cardiovasc Res. 2012. PMID: 22362814 Free PMC article. Review.
-
An updated view on stem cell differentiation into smooth muscle cells.Vascul Pharmacol. 2012 May-Jun;56(5-6):280-7. doi: 10.1016/j.vph.2012.02.014. Epub 2012 Mar 6. Vascul Pharmacol. 2012. PMID: 22421140 Review.
Cited by
-
MicroRNA-320 targeting neuropilin 1 inhibits proliferation and migration of vascular smooth muscle cells and neointimal formation.Int J Med Sci. 2019 Jan 1;16(1):106-114. doi: 10.7150/ijms.28093. eCollection 2019. Int J Med Sci. 2019. PMID: 30662334 Free PMC article.
-
Hallmarks of exosomes.Future Sci OA. 2021 Nov 5;8(1):FSO764. doi: 10.2144/fsoa-2021-0102. eCollection 2022 Jan. Future Sci OA. 2021. PMID: 34900338 Free PMC article. Review.
-
MicroRNA-1 Expression and Function in Hyalomma Anatolicum anatolicum (Acari: Ixodidae) Ticks.Front Physiol. 2021 Apr 8;12:596289. doi: 10.3389/fphys.2021.596289. eCollection 2021. Front Physiol. 2021. PMID: 33897444 Free PMC article.
-
Bone marrow mesenchymal stem cells for post-myocardial infarction cardiac repair: microRNAs as novel regulators.J Cell Mol Med. 2012 Apr;16(4):657-71. doi: 10.1111/j.1582-4934.2011.01471.x. J Cell Mol Med. 2012. PMID: 22004043 Free PMC article. Review.
-
microRNAs: important regulators of stem cells.Stem Cell Res Ther. 2017 May 11;8(1):110. doi: 10.1186/s13287-017-0551-0. Stem Cell Res Ther. 2017. PMID: 28494789 Free PMC article. Review.
References
-
- Lagos-Quintana M. Rauhut R. Lendeckel W. Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. - PubMed
-
- Lagos-Quintana M. Rauhut R. Yalcin A. Meyer J. Lendeckel W. Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–739. - PubMed
-
- Ke XS. Liu CM. Liu DP. Liang CC. MicroRNAs: key participants in gene regulatory networks. Curr Opin Chem Biol. 2003;7:516–523. - PubMed
-
- Hobert O. Common logic of transcription factor and microRNA action. Trends Biochem Sci. 2004;29:462–468. - PubMed
-
- Ji R. Cheng Y. Yue J. Yang J. Liu X. Chen H. Dean DB. Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. - PubMed

