High mobility group box 1 potentiates the pro-inflammatory effects of interleukin-1β in osteoarthritic synoviocytes
- PMID: 20799933
- PMCID: PMC2945068
- DOI: 10.1186/ar3124
High mobility group box 1 potentiates the pro-inflammatory effects of interleukin-1β in osteoarthritic synoviocytes
Abstract
Introduction: High mobility group box 1 (HMGB1) is released by necrotic cells or secreted in response to inflammatory stimuli. Extracellular HMGB1 may act as a pro-inflammatory cytokine in rheumatoid arthritis. We have recently reported that HMGB1 is released by osteoarthritic synoviocytes after activation with interleukin-1beta (IL-1β) The present study investigated the role of HMGB1 in synovial inflammation in osteoarthritis (OA).
Methods: HMGB1 was determined in human synovium using immunohistochemistry, comparing normal to OA. OA synoviocytes were incubated with HMGB1 at 15 or 25 ng/ml in the absence or presence of IL-1β (10 ng/ml). Gene expression was analyzed by quantitative PCR and protein expression by Western Blot and ELISA. Matrix metalloproteinase (MMP) activity was studied by fluorometric procedures and nuclear factor (NF)-κB activation by transient transfection with a NF-κB-luciferase plasmid.
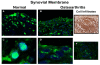
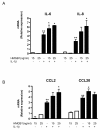
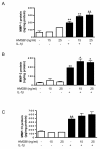
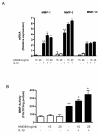
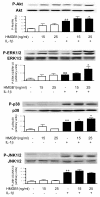
Results: In the normal synovium, HMGB1 was found in the synovial lining cells, sublining cells, and in the vascular wall cells. The distribution of HMGB1 in OA synovium was similar but the number of HMGB1 positive cells was higher and HMGB1 was also present in infiltrated cells. In normal synovial membrane cells, HMGB1 was found mostly in the nuclei, whereas in OA, HMGB1 was generally found mostly in the cytoplasm. In OA synoviocytes, HMGB1 alone at concentrations of 15 or 25 ng/ml did not affect the production of IL-6, IL-8, CCL2, CCL20, MMP-1 or MMP-3, but in the presence of IL-1β, a significant potentiation of protein and mRNA expression, as well as MMP activity was observed. HMGB1 also enhanced the phosphorylated ERK1/2 and p38 levels, with a lower effect on phosphorylated Akt. In contrast, JNK1/2 phosphorylation was not affected. In addition, HMGB1 at 25 ng/ml significantly potentiated NF-κB activation in the presence of IL-1β.
Conclusions: Our results indicate that HMGB1 is overexpressed in OA synovium and mostly present in extracellular form. In OA synoviocytes, HMGB1 cooperates with IL-1β to amplify the inflammatory response leading to the production of a number of cytokines, chemokines and MMPs. Our data support a pro-inflammatory role for this protein contributing to synovitis and articular destruction in OA.
Figures



References
-
- Sundberg E, Grundtman C, af Klint E, Lindberg J, Ernestam S, Ulfgren AK, Harris HE, Andersson U. Systemic TNF blockade does not modulate synovial expression of the pro-inflammatory mediator HMGB1 in rheumatoid arthritis patients - a prospective clinical study. Arthritis Res Ther. 2008;10:R33. doi: 10.1186/ar2387. - DOI - PMC - PubMed
-
- Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

