An analysis of the effects of Mn2+ on oxidative phosphorylation in liver, brain, and heart mitochondria using state 3 oxidation rate assays
- PMID: 20800605
- PMCID: PMC3004221
- DOI: 10.1016/j.taap.2010.08.018
An analysis of the effects of Mn2+ on oxidative phosphorylation in liver, brain, and heart mitochondria using state 3 oxidation rate assays
Abstract
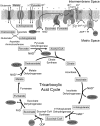
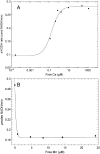
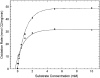
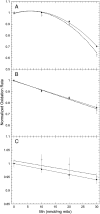
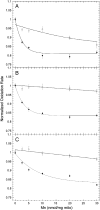
Manganese (Mn) toxicity is partially mediated by reduced ATP production. We have used oxidation rate assays--a measure of ATP production--under rapid phosphorylation conditions to explore sites of Mn(2+) inhibition of ATP production in isolated liver, brain, and heart mitochondria. This approach has several advantages. First, the target tissue for Mn toxicity in the basal ganglia is energetically active and should be studied under rapid phosphorylation conditions. Second, Mn may inhibit metabolic steps which do not affect ATP production rate. This approach allows identification of inhibitions that decrease this rate. Third, mitochondria from different tissues contain different amounts of the components of the metabolic pathways potentially resulting in different patterns of ATP inhibition. Our results indicate that Mn(2+) inhibits ATP production with very different patterns in liver, brain, and heart mitochondria. The primary Mn(2+) inhibition site in liver and heart mitochondria, but not in brain mitochondria, is the F₁F₀ ATP synthase. In mitochondria fueled by either succinate or glutamate+malate, ATP production is much more strongly inhibited in brain than in liver or heart mitochondria; moreover, Mn(2+) inhibits two independent sites in brain mitochondria. The primary site of Mn-induced inhibition of ATP production in brain mitochondria when succinate is substrate is either fumarase or complex II, while the likely site of the primary inhibition when glutamate plus malate are the substrates is either the glutamate/aspartate exchanger or aspartate aminotransferase.
Copyright © 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

