Synthesis and biological evaluation of C-2 halogenated analogs of salvinorin A
- PMID: 20801035
- PMCID: PMC4514444
- DOI: 10.1016/j.bmcl.2010.08.001
Synthesis and biological evaluation of C-2 halogenated analogs of salvinorin A
Abstract
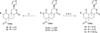
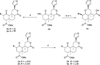
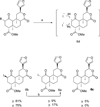
Salvinorin A (1), the main active ingredient of Salvia divinorum, is a potent and selective κ opioid receptor (KOPR) agonist. Based on the SAR, its C-2 position is one of the key binding sites and has very little space tolerance (3-4 carbons atoms) and limited to only lipophilic groups. In our attempt to prepare PET brain imaging agent for mapping KOPR, a series of C-2 halogenated analogs have been synthesized and screened for binding affinity at κ (KOPR), μ (MOPR), and δ (DOPR). These C-2 halogenated analogs with sequential changes of atomic radius and electron density serve as excellent molecular probes for further investigating the binding pocket at C-2, particularly on the effects of α verses β configuration at C-2 position. The results of KOPR binding and functional studies reveal β isomer in general binds better than α isomer with the exception of iodinated analogs and none of the C-2 halogenated analogs shows any improvement of KOPR binding affinity. Interestingly, functional assay has characterized that 6b is a partial agonist with E(max) of 46% of the kappa receptor full agonist U50,488H at 250 nM (K(i)). We have also observed that the affinity to the kappa receptor increases with atomic radius (I>Br>Cl>F) which is in good agreement with halogen bonding interactions reported in the literature.
Copyright © 2010. Published by Elsevier Ltd.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Research Materials
Miscellaneous

