Emerging complex pathways of the actomyosin powerstroke
- PMID: 20801044
- PMCID: PMC2991434
- DOI: 10.1016/j.tibs.2010.07.012
Emerging complex pathways of the actomyosin powerstroke
Abstract
Actomyosin powers muscle contraction and various cellular activities, including cell division, differentiation, intracellular transport and sensory functions. Despite their crucial roles, key aspects of force generation have remained elusive. To perform efficient force generation, the powerstroke must occur while myosin is bound to actin. Paradoxically, this process must be initiated when myosin is in a very low actin-affinity state. Recent results shed light on a kinetic pathway selection mechanism whereby the actin-induced activation of the swing of myosin's lever enables efficient mechanical functioning. Structural elements and biochemical principles involved in this mechanism are conserved among various NTPase-effector (e.g. kinesin-microtubule, G protein exchange factor and kinase-scaffold protein) systems that perform chemomechanical or signal transduction.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

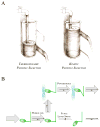
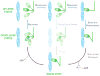

References
-
- Pollard TD. Reflections on a quarter century of research on contractile systems. Trends Biochem Sci. 2000;25:607–611. - PubMed
-
- Geeves MA, Holmes KC. The molecular mechanism of muscle contraction. Adv Protein Chem. 2005;71:161–193. - PubMed
-
- Smith DA, et al. Towards a unified theory of muscle contraction. I: foundations. Ann Biomed Eng. 2008;36:1624–1640. - PubMed
-
- Sweeney HL, Houdusse A. Structural and Functional Insights into the Myosin Motor Mechanism. Annu Rev Biophys. 2010;39:539–557. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

