Brain tumor stem cells: the cancer stem cell hypothesis writ large
- PMID: 20801091
- PMCID: PMC5527933
- DOI: 10.1016/j.molonc.2010.08.001
Brain tumor stem cells: the cancer stem cell hypothesis writ large
Abstract
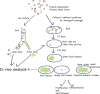
Brain tumors, which are typically very heterogeneous at the cellular level, appear to have a stem cell foundation. Recently, investigations from multiple groups have found that human as well as experimental mouse brain tumors contain subpopulations of cells that functionally behave as tumor stem cells, driving tumor growth and generating tumor cell progeny that form the tumor bulk, but which then lose tumorigenic ability. In human glioblastomas, these tumor stem cells express neural precursor markers and are capable of differentiating into tumor cells that express more mature neural lineage markers. In addition, modeling brain tumors in mice suggests that neural precursor cells more readily give rise to full blown tumors, narrowing potential cells of origin to those rarer brain cells that have a proliferative potential. Applying stem cell concepts and methodologies is giving fresh insight into brain tumor biology, cell of origin and mechanisms of growth, and is offering new opportunities for development of more effective treatments. The field of brain tumor stem cells remains very young and there is much to be learned before these new insights are translated into new patient treatments.
Copyright © 2010 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Bachoo, R.M. , Maher, E.A. , Ligon, K.L. , Sharpless, N.E. , Chan, S.S. , You, M.J. , Tang, Y. , DeFrances, J. , Stover, E. , Weissleder, R. , 2002. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 1, 269–277. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

