Axonal ensheathment and intercellular barrier formation in Drosophila
- PMID: 20801419
- PMCID: PMC4020514
- DOI: 10.1016/S1937-6448(10)83003-5
Axonal ensheathment and intercellular barrier formation in Drosophila
Abstract
Glial cells are critical players in every major aspect of nervous system development, function, and disease. Other than their traditional supportive role, glial cells perform a variety of important functions such as myelination, synapse formation and plasticity, and establishment of blood-brain and blood-nerve barriers in the nervous system. Recent studies highlight the striking functional similarities between Drosophila and vertebrate glia. In both systems, glial cells play an essential role in neural ensheathment thereby isolating the nervous system and help to create a local ionic microenvironment for conduction of nerve impulses. Here, we review the anatomical aspects and the molecular players that underlie ensheathment during different stages of nervous system development in Drosophila and how these processes lead to the organization of neuroglial junctions. We also discuss some key aspects of the invertebrate axonal ensheathment and junctional organization with that of vertebrate myelination and axon-glial interactions. Finally, we highlight the importance of intercellular junctions in barrier formation in various cellular contexts in Drosophila. We speculate that unraveling the genetic and molecular mechanisms of ensheathment across species might provide key insights into human myelin-related disorders and help in designing therapeutic interventions.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
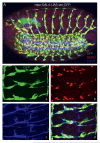
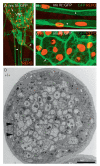
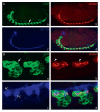

References
-
- Abbott NJ, Lane NJ, Bundgaard M. The blood–brain interface in invertebrates. Ann. N. Y. Acad. Sci. 1986;481:20–42. - PubMed
-
- Akiyama-Oda Y, Hosoya T, Hotta Y. Alteration of cell fate by ectopic expression of Drosophila glial cells missing in non-neural cells. Dev. Genes Evol. 1998;208:578–585. - PubMed
-
- Allen NJ, Barres BA. Neuroscience: glia—more than just brain glue. Nature. 2009;457:675–677. - PubMed
-
- Auld VJ, Fetter RD, Broadie K, Goodman CS. Gliotactin, a novel transmembrane protein on peripheral glia, is required to form the blood–nerve barrier in Drosophila. Cell. 1995;81:757–767. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

