Heart-specific small subunit of myosin light chain phosphatase activates rho-associated kinase and regulates phosphorylation of myosin phosphatase target subunit 1
- PMID: 20801872
- PMCID: PMC2962466
- DOI: 10.1074/jbc.M110.122390
Heart-specific small subunit of myosin light chain phosphatase activates rho-associated kinase and regulates phosphorylation of myosin phosphatase target subunit 1
Abstract
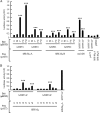
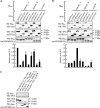
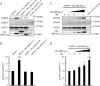
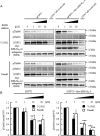
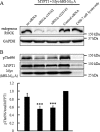
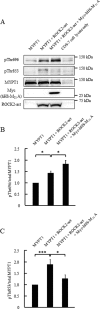
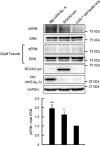
Phosphorylation of myosin regulatory light chain (MLC) plays a regulatory role in muscle contraction, and the level of MLC phosphorylation is balanced by MLC kinase and MLC phosphatase (MLCP). MLCP consists of a catalytic subunit, a large subunit (MYPT1 or MYPT2), and a small subunit. MLCP activity is regulated by phosphorylation of MYPTs, whereas the role of small subunit in the regulation remains unknown. We previously characterized a human heart-specific small subunit (hHS-M(21)) that increased the sensitivity to Ca(2+) in muscle contraction. In this study, we investigated the role of hHS-M(21) in the regulation of MLCP phosphorylation. Two isoforms of hHS-M(21), hHS-M(21)A and hHS-M(21)B, preferentially bound the C-terminal one-third region of MYPT1 and MYPT2, respectively. Amino acid substitutions at a phosphorylation site of MYPT1, Ser-852, impaired the binding of MYPT1 and hHS-M(21). The hHS-M(21) increased the phosphorylation level of MYPT1 at Thr-696, which was attenuated by Rho-associated kinase (ROCK) inhibitors and small interfering RNAs for ROCK. In addition, hHS-M(21) bound ROCK and enhanced the ROCK activity. These findings suggest that hHS-M(21) is a heart-specific effector of ROCK and plays a regulatory role in the MYPT1 phosphorylation at Thr-696 by ROCK.
Figures



References
-
- Kamisoyama H., Araki Y., Ikebe M. (1994) Biochemistry 33, 840–847 - PubMed
-
- Matsumura F., Totsukawa G., Yamakita Y., Yamashiro S. (2001) Cell Struct. Funct. 26, 639–644 - PubMed
-
- Murata-Hori M., Fukuta Y., Ueda K., Iwasaki T., Hosoya H. (2001) Oncogene 20, 8175–8183 - PubMed
-
- Kamm K. E., Stull J. T. (2001) J. Biol. Chem. 276, 4527–4530 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

