Mechanism of activation of PSI-7851 and its diastereoisomer PSI-7977
- PMID: 20801890
- PMCID: PMC2966047
- DOI: 10.1074/jbc.M110.161802
Mechanism of activation of PSI-7851 and its diastereoisomer PSI-7977
Abstract
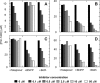
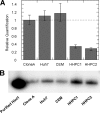
A phosphoramidate prodrug of 2'-deoxy-2'-α-fluoro-β-C-methyluridine-5'-monophosphate, PSI-7851, demonstrates potent anti-hepatitis C virus (HCV) activity both in vitro and in vivo. PSI-7851 is a mixture of two diastereoisomers, PSI-7976 and PSI-7977, with PSI-7977 being the more active inhibitor of HCV RNA replication in the HCV replicon assay. To inhibit the HCV NS5B RNA-dependent RNA polymerase, PSI-7851 must be metabolized to the active triphosphate form. The first step, hydrolysis of the carboxyl ester by human cathepsin A (CatA) and/or carboxylesterase 1 (CES1), is a stereospecific reaction. Western blot analysis showed that CatA and CES1 are both expressed in primary human hepatocytes. However, expression of CES1 is undetectable in clone A replicon cells. Studies with inhibitors of CatA and/or CES1 indicated that CatA is primarily responsible for hydrolysis of the carboxyl ester in clone A cells, although in primary human hepatocytes, both CatA and CES1 contribute to the hydrolysis. Hydrolysis of the ester is followed by a putative nucleophilic attack on the phosphorus by the carboxyl group resulting in the spontaneous elimination of phenol and the production of an alaninyl phosphate metabolite, PSI-352707, which is common to both isomers. The removal of the amino acid moiety of PSI-352707 is catalyzed by histidine triad nucleotide-binding protein 1 (Hint1) to give the 5'-monophosphate form, PSI-7411. siRNA-mediated Hint1 knockdown studies further indicate that Hint1 is, at least in part, responsible for converting PSI-352707 to PSI-7411. PSI-7411 is then consecutively phosphorylated to the diphosphate, PSI-7410, and to the active triphosphate metabolite, PSI-7409, by UMP-CMP kinase and nucleoside diphosphate kinase, respectively.
Figures





Similar articles
-
Activity and the metabolic activation pathway of the potent and selective hepatitis C virus pronucleotide inhibitor PSI-353661.Antiviral Res. 2011 Aug;91(2):120-32. doi: 10.1016/j.antiviral.2011.05.003. Epub 2011 May 12. Antiviral Res. 2011. PMID: 21600932 Free PMC article.
-
Discovery of a β-d-2'-deoxy-2'-α-fluoro-2'-β-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus.J Med Chem. 2010 Oct 14;53(19):7202-18. doi: 10.1021/jm100863x. J Med Chem. 2010. PMID: 20845908
-
PSI-7851, a pronucleotide of beta-D-2'-deoxy-2'-fluoro-2'-C-methyluridine monophosphate, is a potent and pan-genotype inhibitor of hepatitis C virus replication.Antimicrob Agents Chemother. 2010 Aug;54(8):3187-96. doi: 10.1128/AAC.00399-10. Epub 2010 Jun 1. Antimicrob Agents Chemother. 2010. PMID: 20516278 Free PMC article.
-
Pharmacokinetic, Pharmacodynamic, and Drug-Interaction Profile of the Hepatitis C Virus NS5B Polymerase Inhibitor Sofosbuvir.Clin Pharmacokinet. 2015 Jul;54(7):677-90. doi: 10.1007/s40262-015-0261-7. Clin Pharmacokinet. 2015. PMID: 25822283 Review.
-
Sofosbuvir, a novel nucleotide analogue inhibitor used for the treatment of hepatitis C virus.J Pharm Pharmacol. 2014 Dec;66(12):1653-66. doi: 10.1111/jphp.12294. Epub 2014 Aug 31. J Pharm Pharmacol. 2014. PMID: 25175944 Review.
Cited by
-
Meta-analysis of the efficacy and safety of sofosbuvir for the treatment of hepatitis C virus infection.Int J Clin Pharm. 2015 Oct;37(5):698-708. doi: 10.1007/s11096-015-0144-x. Epub 2015 Jun 6. Int J Clin Pharm. 2015. PMID: 26047942 Review.
-
Sofosbuvir for treatment of chronic hepatitis C.Hepatol Int. 2015 Apr;9(2):161-73. doi: 10.1007/s12072-014-9606-9. Epub 2015 Jan 28. Hepatol Int. 2015. PMID: 25788194 Review.
-
PLK1-ELAVL1/HuR-miR-122 signaling facilitates hepatitis C virus proliferation.Proc Natl Acad Sci U S A. 2022 Dec 20;119(51):e2214911119. doi: 10.1073/pnas.2214911119. Epub 2022 Dec 13. Proc Natl Acad Sci U S A. 2022. PMID: 36512502 Free PMC article.
-
Current Status of Direct Acting Antiviral Agents against Hepatitis C Virus Infection in Pakistan.Medicina (Kaunas). 2018 Nov 5;54(5):80. doi: 10.3390/medicina54050080. Medicina (Kaunas). 2018. PMID: 30400604 Free PMC article. Review.
-
Role of Marine Natural Products in the Genesis of Antiviral Agents.Chem Rev. 2015 Sep 23;115(18):9655-706. doi: 10.1021/cr4006318. Epub 2015 Aug 28. Chem Rev. 2015. PMID: 26317854 Free PMC article. Review.
References
-
- Hewlett G., Hallenberger S., Rübsamen-Waigmann H. (2004) Curr. Opin. Pharmacol. 4, 453–464 - PubMed
-
- Naesens L., De Clercq E. (2001) Herpes 8, 12–16 - PubMed
-
- De Clercq E. (2007) Nat. Rev. Drug Discov. 6, 1001–1018 - PubMed
-
- Férir G., Kaptein S., Neyts J., De Clercq E. (2008) Rev. Med. Virol. 18, 19–34 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

