TCDD induced pericardial edema and relative COX-2 expression in medaka (Oryzias Latipes) embryos
- PMID: 20801906
- PMCID: PMC2955216
- DOI: 10.1093/toxsci/kfq254
TCDD induced pericardial edema and relative COX-2 expression in medaka (Oryzias Latipes) embryos
Abstract
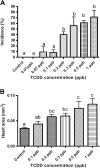
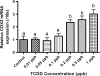
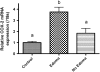
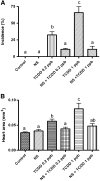
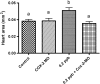
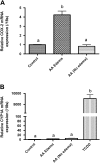
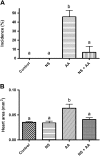
Exposure to dioxin and other aryl hydrocarbon receptor (AhR) ligands results in multiple, specific developmental cardiovascular phenotypes including pericardial edema and circulatory failure in small aquarium fish models. Although phenotypes are well described, mechanistic underpinnings for such toxicities remain elusive. Here we suggest that AhR activation results in stimulation of inflammation and "eicosanoid" pathways, which contribute to the observed developmental, cardiovascular phenotypes. We demonstrate that medaka embryos exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (0.05-1 ppb) during early development result in a dose-related increase in the prevalence of pericardial edema and that this phenotype correlates with an increase in cyclooxygenase-2 (COX-2) gene expression. Those individuals exhibiting the edema phenotype had significantly greater COX-2 mRNA than their nonedematous cohort. Selective pharmacological inhibition of COX-2, with NS-398, and genetic knock down of COX-2 with a translation initiation morpholino significantly attenuated prevalence and severity of edema phenotype. Subsequently, exposures of medaka embryos to arachidonic acid (AA) resulted in recapitulation of the pericardial edema phenotype and significantly increased COX-2 expression only in those individuals exhibiting the edema phenotype compared with their nonedematous cohort. AA exposure does not result in significant induction of cytochrome P450 1A expression, suggesting that pericardial edema can be induced independent of AhR/aryl hydrocarbon receptor nuclear translocator/dioxin response element interactions. Results from this study demonstrate that developmental exposure to TCDD results in an induction of inflammatory mediators including COX-2, which contribute to the onset, and progression of heart dysmorphogenesis in the medaka model.
Figures


References
-
- Antkiewicz DS, Burns CG, Carney SA, Peterson RE, Heideman W. Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol. Sci. 2005;84:368–377. - PubMed
-
- Antkiewicz DS, Peterson RE, Heideman W. Blocking expression of AHR2 and ARNT1 in zebrafish larvae protects against cardiac toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2006;94:175–182. - PubMed
-
- Arzuaga X, Reiterer G, Majkova Z, Kilgore MW, Toborek M, Hennig B. PPARalpha ligands reduce PCB-induced endothelial activation: possible interactions in inflammation and atherosclerosis. Cardiovasc. Toxicol. 2007;7:264–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

