A commensal gone bad: complete genome sequence of the prototypical enterotoxigenic Escherichia coli strain H10407
- PMID: 20802035
- PMCID: PMC2953697
- DOI: 10.1128/JB.00710-10
A commensal gone bad: complete genome sequence of the prototypical enterotoxigenic Escherichia coli strain H10407
Abstract
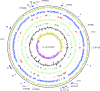
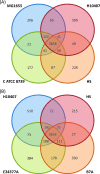
In most cases, Escherichia coli exists as a harmless commensal organism, but it may on occasion cause intestinal and/or extraintestinal disease. Enterotoxigenic E. coli (ETEC) is the predominant cause of E. coli-mediated diarrhea in the developing world and is responsible for a significant portion of pediatric deaths. In this study, we determined the complete genomic sequence of E. coli H10407, a prototypical strain of enterotoxigenic E. coli, which reproducibly elicits diarrhea in human volunteer studies. We performed genomic and phylogenetic comparisons with other E. coli strains, revealing that the chromosome is closely related to that of the nonpathogenic commensal strain E. coli HS and to those of the laboratory strains E. coli K-12 and C. Furthermore, these analyses demonstrated that there were no chromosomally encoded factors unique to any sequenced ETEC strains. Comparison of the E. coli H10407 plasmids with those from several ETEC strains revealed that the plasmids had a mosaic structure but that several loci were conserved among ETEC strains. This study provides a genetic context for the vast amount of experimental and epidemiological data that have been published.
Figures



References
-
- Benjelloun-Touimi, Z., P. J. Sansonetti, and C. Parsot. 1995. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol. Microbiol. 17:123-135. - PubMed
-
- Brown, N. L., S. R. Barrett, J. Camakaris, B. T. Lee, and D. A. Rouch. 1995. Molecular genetics and transport analysis of the copper-resistance determinant (pco) from Escherichia coli plasmid pRJ1004. Mol. Microbiol. 17:1153-1166. - PubMed
-
- Chaudhuri, R. R., M. Sebaihia, J. L. Hobman, M. A. Webber, D. L. Leyton, M. D. Goldberg, A. F. Cunningham, A. Scott-Tucker, P. R. Ferguson, C. M. Thomas, G. Frankel, C. M. Tang, E. G. Dudley, I. S. Roberts, D. A. Rasko, M. J. Pallen, J. Parkhill, J. P. Nataro, N. R. Thomson, and I. R. Henderson. 2010. Complete genome sequence and comparative metabolic profiling of the prototypical enteroaggregative Escherichia coli strain 042. PLoS One 5:e8801. - PMC - PubMed
-
- Chen, Q., S. J. Savarino, and M. M. Venkatesan. 2006. Subtractive hybridization and optical mapping of the enterotoxigenic Escherichia coli H10407 chromosome: isolation of unique sequences and demonstration of significant similarity to the chromosome of E. coli K-12. Microbiology 152:1041-1054. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

