Comparative analysis of the biochemical and functional properties of C-terminal domains of autotransporters
- PMID: 20802036
- PMCID: PMC2953703
- DOI: 10.1128/JB.00432-10
Comparative analysis of the biochemical and functional properties of C-terminal domains of autotransporters
Abstract
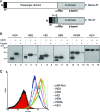
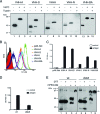
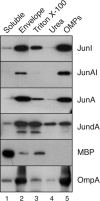
Autotransporters (ATs) are the largest group of proteins secreted by Gram-negative bacteria and include many virulence factors from human pathogens. ATs are synthesized as large precursors with a C-terminal domain that is inserted in the outer membrane (OM) and is essential for the translocation of an N-terminal passenger domain to the extracellular milieu. Several mechanisms have been proposed for AT secretion. Self-translocation models suggest transport across a hydrophilic channel formed by an internal pore of the β-barrel or by the oligomerization of C-terminal domains. Alternatively, an assisted-translocation model suggests that transport employs a conserved machinery of the bacterial OM such as the Bam complex. In this work we have investigated AT secretion by carrying out a comparative study to analyze the conserved biochemical and functional features of different C-terminal domains selected from ATs of gammaproteobacteria, betaproteobacteria, alphaproteobacteria, and epsilonproteobacteria. Our results indicate that C-terminal domains having an N-terminal α-helix and a β-barrel constitute functional transport units for the translocation of peptides and immunoglobulin domains with disulfide bonds. In vivo and in vitro analyses show that multimerization is not a conserved feature in AT C-terminal domains. Furthermore, we demonstrate that the deletion of the conserved α-helix severely impairs β-barrel folding and OM insertion and thereby blocks passenger domain secretion. These observations suggest that the AT β-barrel without its α-helix cannot form a stable hydrophilic channel in the OM for protein translocation. The implications of our data for an understanding of AT secretion are discussed.
Figures




Similar articles
-
Autotransporter β-domains have a specific function in protein secretion beyond outer-membrane targeting.J Mol Biol. 2011 Sep 30;412(4):553-67. doi: 10.1016/j.jmb.2011.07.035. Epub 2011 Jul 23. J Mol Biol. 2011. PMID: 21806993
-
The Bam (Omp85) complex is involved in secretion of the autotransporter haemoglobin protease.Microbiology (Reading). 2009 Dec;155(Pt 12):3982-3991. doi: 10.1099/mic.0.034991-0. Epub 2009 Oct 8. Microbiology (Reading). 2009. PMID: 19815580
-
Molecular basis for the folding of β-helical autotransporter passenger domains.Nat Commun. 2018 Apr 11;9(1):1395. doi: 10.1038/s41467-018-03593-2. Nat Commun. 2018. PMID: 29643377 Free PMC article.
-
Job contenders: roles of the β-barrel assembly machinery and the translocation and assembly module in autotransporter secretion.Mol Microbiol. 2017 Nov;106(4):505-517. doi: 10.1111/mmi.13832. Epub 2017 Sep 26. Mol Microbiol. 2017. PMID: 28887826 Review.
-
Looks can be deceiving: recent insights into the mechanism of protein secretion by the autotransporter pathway.Mol Microbiol. 2015 Jul;97(2):205-15. doi: 10.1111/mmi.13031. Epub 2015 May 15. Mol Microbiol. 2015. PMID: 25881492 Free PMC article. Review.
Cited by
-
Functional cell surface display and controlled secretion of diverse Agarolytic enzymes by Escherichia coli with a novel ligation-independent cloning vector based on the autotransporter YfaL.Appl Environ Microbiol. 2012 May;78(9):3051-8. doi: 10.1128/AEM.07004-11. Epub 2012 Feb 17. Appl Environ Microbiol. 2012. PMID: 22344647 Free PMC article.
-
Escherichia coli surface display for the selection of nanobodies.Microb Biotechnol. 2017 Nov;10(6):1468-1484. doi: 10.1111/1751-7915.12819. Epub 2017 Aug 3. Microb Biotechnol. 2017. PMID: 28772027 Free PMC article. Review.
-
Comparing autotransporter β-domain configurations for their capacity to secrete heterologous proteins to the cell surface.PLoS One. 2018 Feb 7;13(2):e0191622. doi: 10.1371/journal.pone.0191622. eCollection 2018. PLoS One. 2018. PMID: 29415042 Free PMC article.
-
Programming controlled adhesion of E. coli to target surfaces, cells, and tumors with synthetic adhesins.ACS Synth Biol. 2015 Apr 17;4(4):463-73. doi: 10.1021/sb500252a. Epub 2014 Jul 29. ACS Synth Biol. 2015. PMID: 25045780 Free PMC article.
-
From self sufficiency to dependence: mechanisms and factors important for autotransporter biogenesis.Nat Rev Microbiol. 2012 Feb 16;10(3):213-25. doi: 10.1038/nrmicro2733. Nat Rev Microbiol. 2012. PMID: 22337167 Review.
References
-
- Bernstein, H. D. 2007. Are bacterial ‘autotransporters’ really transporters? Trends Microbiol. 15:441-447. - PubMed
-
- Bitto, E., and D. B. McKay. 2003. The periplasmic molecular chaperone protein SurA binds a peptide motif that is characteristic of integral outer membrane proteins. J. Biol. Chem. 278:49316-49322. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

