Folic acid mitigated cardiac dysfunction by normalizing the levels of tissue inhibitor of metalloproteinase and homocysteine-metabolizing enzymes postmyocardial infarction in mice
- PMID: 20802128
- PMCID: PMC2993194
- DOI: 10.1152/ajpheart.00577.2010
Folic acid mitigated cardiac dysfunction by normalizing the levels of tissue inhibitor of metalloproteinase and homocysteine-metabolizing enzymes postmyocardial infarction in mice
Abstract
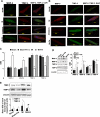
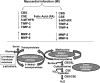
Myocardial infarction (MI) results in significant metabolic derangement, causing accumulation of metabolic by product, such as homocysteine (Hcy). Hcy is a nonprotein amino acid generated during nucleic acid methylation and demethylation of methionine. Folic acid (FA) decreases Hcy levels by remethylating the Hcy to methionine, by 5-methylene tetrahydrofolate reductase (5-MTHFR). Although clinical trials were inconclusive regarding the role of Hcy in MI, in animal models, the levels of 5-MTHFR were decreased, and FA mitigated the MI injury. We hypothesized that FA mitigated MI-induced injury, in part, by mitigating cardiac remodeling during chronic heart failure. Thus, MI was induced in 12-wk-old male C57BL/J mice by ligating the left anterior descending artery, and FA (0.03 g/l in drinking water) was administered for 4 wk after the surgery. Cardiac function was assessed by echocardiography and by a Millar pressure-volume catheter. The levels of Hcy-metabolizing enzymes, cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 5-MTHFR, were estimated by Western blot analyses. The results suggest that FA administered post-MI significantly improved cardiac ejection fraction and induced tissue inhibitor of metalloproteinase, CBS, CSE, and 5-MTHFR. We showed that FA supplementation resulted in significant improvement of myocardial function after MI. The study eluted the importance of homocysteine (Hcy) metabolism and FA supplementation in cardiovascular disease.
Figures






Similar articles
-
Attenuation of beta2-adrenergic receptors and homocysteine metabolic enzymes cause diabetic cardiomyopathy.Biochem Biophys Res Commun. 2010 Oct 15;401(2):175-81. doi: 10.1016/j.bbrc.2010.09.006. Epub 2010 Sep 15. Biochem Biophys Res Commun. 2010. PMID: 20836991 Free PMC article.
-
Folic acid improves inner ear vascularization in hyperhomocysteinemic mice.Hear Res. 2012 Feb;284(1-2):42-51. doi: 10.1016/j.heares.2011.12.006. Epub 2011 Dec 24. Hear Res. 2012. PMID: 22222235 Free PMC article.
-
Reversal of endocardial endothelial dysfunction by folic acid in homocysteinemic hypertensive rats.Am J Hypertens. 2002 Feb;15(2 Pt 1):157-63. doi: 10.1016/s0895-7061(01)02286-5. Am J Hypertens. 2002. PMID: 11863251
-
Homocysteine and Hyperhomocysteinaemia.Curr Med Chem. 2019;26(16):2948-2961. doi: 10.2174/0929867325666180313105949. Curr Med Chem. 2019. PMID: 29532755 Review.
-
Homocysteine and DNA methylation: a review of animal and human literature.Mol Genet Metab. 2014 Dec;113(4):243-52. doi: 10.1016/j.ymgme.2014.10.006. Epub 2014 Oct 14. Mol Genet Metab. 2014. PMID: 25456744 Review.
Cited by
-
Tetrahydrocurcumin ameliorates homocysteinylated cytochrome-c mediated autophagy in hyperhomocysteinemia mice after cerebral ischemia.J Mol Neurosci. 2012 May;47(1):128-38. doi: 10.1007/s12031-011-9695-z. Epub 2012 Jan 3. J Mol Neurosci. 2012. PMID: 22212488 Free PMC article.
-
Hydrogen sulfide mitigates cardiac remodeling during myocardial infarction via improvement of angiogenesis.Int J Biol Sci. 2012;8(4):430-41. doi: 10.7150/ijbs.3632. Epub 2012 Feb 28. Int J Biol Sci. 2012. PMID: 22419888 Free PMC article.
-
Folate-Dependent Cognitive Impairment Associated With Specific Gene Networks in the Adult Mouse Hippocampus.Front Nutr. 2020 Nov 12;7:574730. doi: 10.3389/fnut.2020.574730. eCollection 2020. Front Nutr. 2020. PMID: 33282900 Free PMC article.
-
Autophagy mechanism of right ventricular remodeling in murine model of pulmonary artery constriction.Am J Physiol Heart Circ Physiol. 2012 Feb 1;302(3):H688-96. doi: 10.1152/ajpheart.00777.2011. Epub 2011 Nov 18. Am J Physiol Heart Circ Physiol. 2012. PMID: 22101525 Free PMC article.
-
The Roles of Aerobic Exercise and Folate Supplementation in Hyperhomocysteinemia-Accelerated Atherosclerosis.Acta Cardiol Sin. 2023 Mar;39(2):309-318. doi: 10.6515/ACS.202303_39(2).20221027A. Acta Cardiol Sin. 2023. PMID: 36911543 Free PMC article.
References
-
- Antoniades C, Shirodaria C, Warrick N, Cai S, de Bono J, Lee J, Leeson P, Neubauer S, Ratnatunga C, Pillai R, Refsum H, Channon KM. 5-Methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation 114: 1193– 1201, 2006 - PubMed
-
- Boushey A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. J Am Med Assoc 274: 1049–1057, 1995 - PubMed
-
- Butler GS, Apte SS, Willenbrock F, Murphy G. Human tissue inhibitor of metalloproteinases 3 interacts with both the N- and C-terminal domains of gelatinases A and B. J Biol Chem 274: 10846– 10851, 1999 - PubMed
-
- Carnicer RA, Navarro M, Arbonés-Mainar JM, Acín S, Guzmán MA, Surra JC, Arnal C, de las Heras M, Blanco-Vaca F, Osada J. Folic acid supplementation delays atherosclerotic lesion development in apoE-deficient mice. Life Sci 80: 638– 643, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

