Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia
- PMID: 20802146
- PMCID: PMC3428148
- DOI: 10.4049/jimmunol.1000803
Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia
Abstract
Endotoxin administration recapitulates many of the host responses to sepsis. Inhibitors of the cysteine protease caspase 1 have long been sought as a therapeutic because mice lacking caspase 1 are resistant to LPS-induced endotoxic shock. According to current thinking, caspase 1-mediated shock requires the proinflammatory caspase 1 substrates IL-1β and IL-18. We show, however, that mice lacking both IL-1β and IL-18 are normally susceptible to LPS-induced splenocyte apoptosis and endotoxic shock. This finding indicates the existence of another caspase 1-dependent mediator of endotoxemia. Reduced serum high mobility group box 1 (HMGB1) levels in caspase 1-deficient mice correlated with their resistance to LPS. A critical role for HMGB1 in endotoxemia was confirmed when mice deficient for IL-1β and IL-18 were protected from a lethal dose of LPS by pretreatment with HMGB1-neutralizing Abs. We found that HMGB1 secretion from LPS-primed macrophages required the inflammasome components apoptotic speck protein containing a caspase activation and recruitment domain (ASC), caspase 1 and Nalp3, whereas HMGB1 secretion from macrophages infected in vitro with Salmonella typhimurium was dependent on caspase 1 and Ipaf. Thus, HMGB1 secretion, which is critical for endotoxemia, occurs downstream of inflammasome assembly and caspase 1 activation.
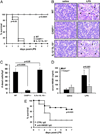
Figures




References
-
- Gautier EL, Huby T, Saint-Charles F, Ouzilleau B, Chapman MJ, Lesnik P. Enhanced dendritic cell survival attenuates lipopolysaccharide-induced immunosuppression and increases resistance to lethal endotoxic shock. J. Immunol. 2008;180:6941–6946. - PubMed
-
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. - PubMed
-
- Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J. Immunol. 2000;165:2950–2954. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

