Sox17 modulates Wnt3A/beta-catenin-mediated transcriptional activation of the Lef-1 promoter
- PMID: 20802155
- PMCID: PMC2980392
- DOI: 10.1152/ajplung.00140.2010
Sox17 modulates Wnt3A/beta-catenin-mediated transcriptional activation of the Lef-1 promoter
Abstract
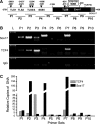
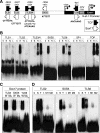
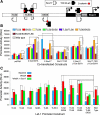
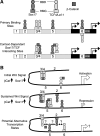
Wnt/β-catenin-dependent activation of lymphoid enhancer factor 1 (Lef-1) plays an important role in numerous developmental processes. In this context, transcription of the Lef-1 gene is increased by Wnt-mediated TCF4/β-catenin activation on the Lef-1 promoter through mechanisms that remain poorly defined. In mouse airway submucosal gland progenitor cells, Wnt3A transiently induces Lef-1 gene expression, and this process is required for epithelial cell proliferation and glandular morphogenesis. In the present study, we sought to identify additional candidate transcriptional regulators of the Lef-1 gene during glandular morphogenesis. To this end, we found that Sox17 expression is dramatically downregulated in early glandular progenitor cells that induce Lef-1 expression. Wnt stimulation of undifferentiated primary airway epithelial cells induced similar changes in Sox17 and Lef-1 expression. Reporter assays revealed that ectopic expression of Sox17 suppresses Wnt3A/β-catenin activation of the Lef-1 promoter in cell lines. EMSA and ChIP analyses defined several Sox17- and TCF4-binding sites that collaborate in transcriptional control of the Lef-1 promoter. More specifically, Sox17 bound to four sites in the Lef-1 promoter, either directly or indirectly through TCF complexes. The DNA- or β-catenin-binding domains of Sox17 controlled context-specific binding of Sox17/TCF complexes on the Lef-1 promoter. Combinatorial site-directed mutagenesis of Sox17- or TCF-binding sites in the Lef-1 promoter demonstrated that these sites control Wnt/β-catenin-mediated induction and/or repression. These findings demonstrate for the first time that Sox17 can directly regulate Wnt/β-catenin-dependent transcription of the Lef-1 promoter and reveal new context-dependent binding sites in the Lef-1 promoter that facilitate protein-protein interactions between Sox17 and TCF4.
Figures






Similar articles
-
Wnt3a regulates Lef-1 expression during airway submucosal gland morphogenesis.Dev Biol. 2007 May 1;305(1):90-102. doi: 10.1016/j.ydbio.2007.01.038. Epub 2007 Feb 7. Dev Biol. 2007. PMID: 17335794 Free PMC article.
-
Down-regulation of Sox7 is associated with aberrant activation of Wnt/b-catenin signaling in endometrial cancer.Oncotarget. 2012 Dec;3(12):1546-56. doi: 10.18632/oncotarget.667. Oncotarget. 2012. PMID: 23295859 Free PMC article.
-
Sox17 and Sox4 differentially regulate beta-catenin/T-cell factor activity and proliferation of colon carcinoma cells.Mol Cell Biol. 2007 Nov;27(22):7802-15. doi: 10.1128/MCB.02179-06. Epub 2007 Sep 17. Mol Cell Biol. 2007. PMID: 17875931 Free PMC article.
-
Cell-context dependent TCF/LEF expression and function: alternative tales of repression, de-repression and activation potentials.Crit Rev Eukaryot Gene Expr. 2011;21(3):207-36. doi: 10.1615/critreveukargeneexpr.v21.i3.10. Crit Rev Eukaryot Gene Expr. 2011. PMID: 22111711 Free PMC article. Review.
-
Inhibition of nuclear Wnt signalling: challenges of an elusive target for cancer therapy.Br J Pharmacol. 2017 Dec;174(24):4589-4599. doi: 10.1111/bph.13963. Epub 2017 Aug 24. Br J Pharmacol. 2017. PMID: 28752891 Free PMC article. Review.
Cited by
-
Conditional deletion of Sox17 reveals complex effects on uterine adenogenesis and function.Dev Biol. 2016 Jun 15;414(2):219-27. doi: 10.1016/j.ydbio.2016.04.010. Epub 2016 Apr 19. Dev Biol. 2016. PMID: 27102016 Free PMC article.
-
Global transcriptional profiling reveals distinct functions of thymic stromal subsets and age-related changes during thymic involution.Cell Rep. 2014 Oct 9;9(1):402-415. doi: 10.1016/j.celrep.2014.08.070. Epub 2014 Oct 2. Cell Rep. 2014. PMID: 25284794 Free PMC article.
-
Knockdown of miR-194-5p inhibits cell proliferation, migration and invasion in breast cancer by regulating the Wnt/β-catenin signaling pathway.Int J Mol Med. 2018 Dec;42(6):3355-3363. doi: 10.3892/ijmm.2018.3897. Epub 2018 Sep 25. Int J Mol Med. 2018. PMID: 30272253 Free PMC article.
-
Predicting and validating the pathway of Wnt3a-driven suppression of osteoclastogenesis.Cell Signal. 2014 Nov;26(11):2358-69. doi: 10.1016/j.cellsig.2014.07.018. Epub 2014 Jul 16. Cell Signal. 2014. PMID: 25038457 Free PMC article.
-
Sox2 modulates Lef-1 expression during airway submucosal gland development.Am J Physiol Lung Cell Mol Physiol. 2014 Apr 1;306(7):L645-60. doi: 10.1152/ajplung.00157.2013. Epub 2014 Jan 31. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24487391 Free PMC article.
References
-
- Anderson RD, Haskell RE, Xia H, Roessler BJ, Davidson BL. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther 7: 1034–1038, 2000 - PubMed
-
- Arce L, Yokoyama NN, Waterman ML. Diversity of LEF/TCF action in development and disease. Oncogene 25: 7492–7504, 2006 - PubMed
-
- Atcha FA, Munguia JE, Li TW, Hovanes K, Waterman ML. A new beta-catenin-dependent activation domain in T cell factor. J Biol Chem 278: 16169–16175, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

