A{beta} accelerates the spatiotemporal progression of tau pathology and augments tau amyloidosis in an Alzheimer mouse model
- PMID: 20802182
- PMCID: PMC2947292
- DOI: 10.2353/ajpath.2010.100346
A{beta} accelerates the spatiotemporal progression of tau pathology and augments tau amyloidosis in an Alzheimer mouse model
Abstract
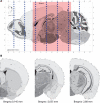
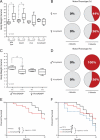
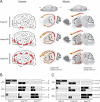
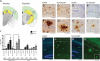
Senile plaques formed by β-amyloid peptides (Aβ) and neurofibrillary tangles (NFTs) formed by hyperphosphorylated tau, a microtubule-associated protein, are the hallmark lesions of Alzheimer's disease (AD) in addition to loss of neurons. While several transgenic (Tg) mouse models have recapitulated aspects of AD-like Aβ and tau pathologies, a spatiotemporal mapping paradigm for progressive NFT accumulation is urgently needed to stage disease progression in AD mouse models. Braak and co-workers developed an effective and widely used NFT staging paradigm for human AD brains. The creation of a Braak-like spatiotemporal staging scheme for tau pathology in mouse models would facilitate mechanistic studies of AD-like tau pathology. Such a scheme would also enhance the reproducibility of preclinical AD therapeutic studies. Thus, we developed a novel murine model of Aβ and tau pathologies and devised a spatiotemporal scheme to stage the emergence and accumulation of NFTs with advancing age. Notably, the development of NFTs followed a spatiotemporal Braak-like pattern similar to that observed in authentic AD. More significantly, the presence of Aβ accelerated NFT formation and enhanced tau amyloidosis; however, tau pathology did not have the same effect on Aβ pathology. This novel NFT staging scheme provides new insights into the mechanisms of tau pathobiology, and we speculate that this scheme will prove useful for other basic and translational studies of AD mouse models.
Figures


Comment in
-
Braak staging in mouse models of Alzheimer's disease.Am J Pathol. 2010 Oct;177(4):1603-5. doi: 10.2353/ajpath.2010.100656. Epub 2010 Sep 2. Am J Pathol. 2010. PMID: 20813967 Free PMC article.
References
-
- Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. - PubMed
-
- Bondareff W, Mountjoy CQ, Roth M, Hauser DL. Neurofibrillary degeneration and neuronal loss in Alzheimer's disease. Neurobiol Aging. 1989;10:709–715. - PubMed
-
- Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, Parisi JE, Hyman BT. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Ann Neurol. 1997;41:17–24. - PubMed
-
- Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. - PubMed
-
- Schonheit B, Zarski R, Ohm TG. Spatial and temporal relationships between plaques and tangles in Alzheimer-pathology. Neurobiol Aging. 2004;25:697–711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

