CLE peptides can negatively regulate protoxylem vessel formation via cytokinin signaling
- PMID: 20802224
- PMCID: PMC3023848
- DOI: 10.1093/pcp/pcq129
CLE peptides can negatively regulate protoxylem vessel formation via cytokinin signaling
Abstract
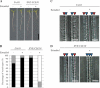
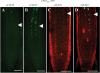
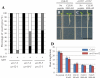
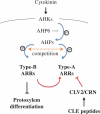
Cell-cell communication is critical for tissue and organ development. In plants, secretory CLAVATA3/EMBRYO SURROUNDING REGION-related (CLE) peptides function as intercellular signaling molecules in various aspects of tissue development including vascular development. However, little is known about intracellular signaling pathways functioning in vascular development downstream of the CLE ligands. We show that CLE peptides including CLE10, which is preferentially expressed in the root vascular system, inhibit protoxylem vessel formation in Arabidopsis roots. GeneChip analysis displayed that CLE10 peptides repressed specifically the expression of two type-A Arabidopsis Response Regulators (ARRs), ARR5 and ARR6, whose products act as negative regulators of cytokinin signaling. The arr5 arr6 roots exhibited defective protoxylem vessel formation. These results indicate that CLE10 inhibits protoxylem vessel formation by suppressing the expression of type-A ARR genes including ARR5 and ARR6. This was supported by the finding that CLE10 did not suppress protoxylem vessel formation in a background of arr10 arr12, a double mutant of type-B ARR genes. Thus, our results revealed cross-talk between CLE signaling and cytokinin signaling in protoxylem vessel formation in roots. Taken together with the indication that cytokinin signaling functions downstream of the CLV3/WUS signaling pathway in the shoot apical meristem, the cross-talk between CLE and cytokinin signaling pathways may be a common feature in plant development.
Figures






Similar articles
-
Type-B ARR transcription factors, ARR10 and ARR12, are implicated in cytokinin-mediated regulation of protoxylem differentiation in roots of Arabidopsis thaliana.Plant Cell Physiol. 2007 Jan;48(1):84-96. doi: 10.1093/pcp/pcl040. Epub 2006 Nov 27. Plant Cell Physiol. 2007. PMID: 17132632
-
Type B response regulators of Arabidopsis play key roles in cytokinin signaling and plant development.Plant Cell. 2008 Aug;20(8):2102-16. doi: 10.1105/tpc.108.059584. Epub 2008 Aug 22. Plant Cell. 2008. PMID: 18723577 Free PMC article.
-
The Arabidopsis eukaryotic translation initiation factor eIF5A-2 regulates root protoxylem development by modulating cytokinin signaling.Plant Cell. 2013 Oct;25(10):3841-57. doi: 10.1105/tpc.113.116236. Epub 2013 Oct 25. Plant Cell. 2013. PMID: 24163315 Free PMC article.
-
CLE signaling systems during plant development and nematode infection.Plant Cell Physiol. 2012 Dec;53(12):1989-99. doi: 10.1093/pcp/pcs136. Epub 2012 Oct 8. Plant Cell Physiol. 2012. PMID: 23045524 Review.
-
CLE peptides and their signaling pathways in plant development.J Exp Bot. 2016 Aug;67(16):4813-26. doi: 10.1093/jxb/erw208. Epub 2016 May 26. J Exp Bot. 2016. PMID: 27229733 Review.
Cited by
-
Absorption, translocation, and effects of Bt Cry1Ac peptides from transgenic cotton to the intercrops and soil functional bacteria.Sci Rep. 2020 Oct 14;10(1):17294. doi: 10.1038/s41598-020-73375-8. Sci Rep. 2020. PMID: 33057018 Free PMC article.
-
Suppression of Arabidopsis protophloem differentiation and root meristem growth by CLE45 requires the receptor-like kinase BAM3.Proc Natl Acad Sci U S A. 2013 Apr 23;110(17):7074-9. doi: 10.1073/pnas.1222314110. Epub 2013 Apr 8. Proc Natl Acad Sci U S A. 2013. PMID: 23569225 Free PMC article.
-
Border sequences of Medicago truncatula CLE36 are specifically cleaved by endoproteases common to the extracellular fluids of Medicago and soybean.J Exp Bot. 2011 Aug;62(13):4649-59. doi: 10.1093/jxb/err185. Epub 2011 Jun 1. J Exp Bot. 2011. PMID: 21633083 Free PMC article.
-
A major role of class III HD-ZIPs in promoting sugar beet cyst nematode parasitism in Arabidopsis.PLoS Pathog. 2024 Nov 7;20(11):e1012610. doi: 10.1371/journal.ppat.1012610. eCollection 2024 Nov. PLoS Pathog. 2024. PMID: 39509386 Free PMC article.
-
CLE2 regulates light-dependent carbohydrate metabolism in Arabidopsis shoots.Plant Mol Biol. 2020 Dec;104(6):561-574. doi: 10.1007/s11103-020-01059-y. Epub 2020 Sep 27. Plant Mol Biol. 2020. PMID: 32980951
References
-
- Butenko M.A., Vie A.K., Brembu T., Aalen R.B., Bones A.M. Plant peptides in signalling: looking for new partners. Trends Plant Sci. 2009;14:255–263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

