β-arrestin Kurtz inhibits MAPK and Toll signalling in Drosophila development
- PMID: 20802461
- PMCID: PMC2957207
- DOI: 10.1038/emboj.2010.202
β-arrestin Kurtz inhibits MAPK and Toll signalling in Drosophila development
Abstract
β-Arrestins have been implicated in the regulation of multiple signalling pathways. However, their role in organism development is not well understood. In this study, we report a new in vivo function of the Drosophila β-arrestin Kurtz (Krz) in the regulation of two distinct developmental signalling modules: MAPK ERK and NF-κB, which transmit signals from the activated receptor tyrosine kinases (RTKs) and the Toll receptor, respectively. Analysis of the expression of effectors and target genes of Toll and the RTK Torso in krz maternal mutants reveals that Krz limits the activity of both pathways in the early embryo. Protein interaction studies suggest a previously uncharacterized mechanism for ERK inhibition: Krz can directly bind and sequester an inactive form of ERK, thus preventing its activation by the upstream kinase, MEK. A simultaneous dysregulation of different signalling systems in krz mutants results in an abnormal patterning of the embryo and severe developmental defects. Our findings uncover a new in vivo function of β-arrestins and present a new mechanism of ERK inhibition by the Drosophila β-arrestin Krz.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
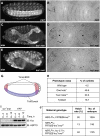

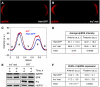


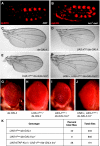
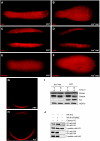
References
-
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT (1999) Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286: 2495–2498 - PubMed
-
- Brunner D, Oellers N, Szabad J, Biggs WH III, Zipursky SL, Hafen E (1994) A gain-of-function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathways. Cell 76: 875–888 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

