Disulphide production by Ero1α-PDI relay is rapid and effectively regulated
- PMID: 20802462
- PMCID: PMC2957208
- DOI: 10.1038/emboj.2010.203
Disulphide production by Ero1α-PDI relay is rapid and effectively regulated
Abstract
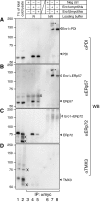
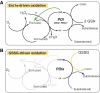
The molecular networks that control endoplasmic reticulum (ER) redox conditions in mammalian cells are incompletely understood. Here, we show that after reductive challenge the ER steady-state disulphide content is restored on a time scale of seconds. Both the oxidase Ero1α and the oxidoreductase protein disulphide isomerase (PDI) strongly contribute to the rapid recovery kinetics, but experiments in ERO1-deficient cells indicate the existence of parallel pathways for disulphide generation. We find PDI to be the main substrate of Ero1α, and mixed-disulphide complexes of Ero1 primarily form with PDI, to a lesser extent with the PDI-family members ERp57 and ERp72, but are not detectable with another homologue TMX3. We also show for the first time that the oxidation level of PDIs and glutathione is precisely regulated. Apparently, this is achieved neither through ER import of thiols nor by transport of disulphides to the Golgi apparatus. Instead, our data suggest that a dynamic equilibrium between Ero1- and glutathione disulphide-mediated oxidation of PDIs constitutes an important element of ER redox homeostasis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- Appenzeller-Herzog C, Ellgaard L (2008a) In vivo reduction-oxidation state of protein disulfide isomerase: the two active sites independently occur in the reduced and oxidized forms. Antioxid Redox Signal 10: 55–64 - PubMed
-
- Appenzeller-Herzog C, Ellgaard L (2008b) The human PDI family: versatility packed into a single fold. Biochim Biophys Acta 1783: 535–548 - PubMed
-
- Banhegyi G, Lusini L, Puskas F, Rossi R, Fulceri R, Braun L, Mile V, di Simplicio P, Mandl J, Benedetti A (1999) Preferential transport of glutathione versus glutathione disulfide in rat liver microsomal vesicles. J Biol Chem 274: 12213–12216 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

