Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling
- PMID: 20802829
- PMCID: PMC2925075
- DOI: 10.1128/mBio.00102-10
Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling
Abstract
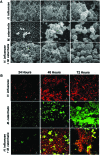
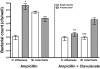
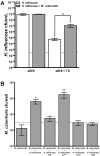
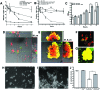
Otitis media (OM) is among the leading diseases of childhood and is caused by opportunists that reside within the nasopharynx, such as Haemophilus influenzae and Moraxella catarrhalis. As with most airway infections, it is now clear that OM infections involve multiple organisms. This study addresses the hypothesis that polymicrobial infection alters the course, severity, and/or treatability of OM disease. The results clearly show that coinfection with H. influenzae and M. catarrhalis promotes the increased resistance of biofilms to antibiotics and host clearance. Using H. influenzae mutants with known biofilm defects, these phenotypes were shown to relate to biofilm maturation and autoinducer-2 (AI-2) quorum signaling. In support of the latter mechanism, chemically synthesized AI-2 (dihydroxypentanedione [DPD]) promoted increased M. catarrhalis biofilm formation and resistance to antibiotics. In the chinchilla infection model of OM, polymicrobial infection promoted M. catarrhalis persistence beyond the levels seen in animals infected with M. catarrhalis alone. Notably, no such enhancement of M. catarrhalis persistence was observed in animals infected with M. catarrhalis and a quorum signaling-deficient H. influenzae luxS mutant strain. We thus conclude that H. influenzae promotes M. catarrhalis persistence within polymicrobial biofilms via interspecies quorum signaling. AI-2 may therefore represent an ideal target for disruption of chronic polymicrobial infections. Moreover, these results strongly imply that successful vaccination against the unencapsulated H. influenzae strains that cause airway infections may also significantly impact chronic M. catarrhalis disease by removing a reservoir of the AI-2 signal that promotes M. catarrhalis persistence within biofilm.
Figures
References
-
- Klein J. O. 2000. The burden of otitis media. Vaccine 19:S2–S8 - PubMed
-
- Paradise J. L., Rockette H. E., Colborn D. K., Bernard B. S., Smith C. G., Kurs-Lasky M., Janosky J. E. 1997. Otitis media in 2253 Pittsburgh-area infants: prevalence and risk factors during the first two years of life. Pediatrics 99:318–333 - PubMed
-
- Finkelstein J. A., Davis R. L., Dowell S. F., Metlay J. P., Soumerai S. B., Rifas-Shiman S. L., Higham M., Miller Z., Miroshnik I., Pedan A., Platt R. 2001. Reducing antibiotic use in children: a randomized trial in 12 practices. Pediatrics 108:1–7 - PubMed
-
- Pichichero M. E. 2000. Recurrent and persistent otitis media. Pediatr. Infect. Dis. J. 19:911–916 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
