Concise Preparation of Novel Tricyclic Chemotypes: Fused Hydantoin-benzodiazepines
- PMID: 20802841
- PMCID: PMC2928152
- DOI: 10.1016/j.tetlet.2010.06.131
Concise Preparation of Novel Tricyclic Chemotypes: Fused Hydantoin-benzodiazepines
Abstract
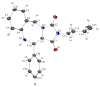
The following article describes a concise synthesis of a collection of 4,5-dihydro-1H-benzo[e][1,4]diazepines fused to a hydantoin ring. Molecular complexity and biological relevance is high and structures are generated in a mere three steps, employing the Ugi reaction to assemble diversity reagents. The protocol represents a novel UDC (Ugi-deprotect-cyclize) strategy employed in the Ugi-5-component CO(2) mediated condensation, followed by further cyclization under basic conditions, to afford the fused hydantoin. Mechanistic caveats, dependent on aldehydes of choice will be revealed and a facile oxidation of final products to imidazolidenetriones briefly discussed.
Figures

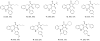


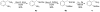
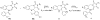

References
-
- Hulme C. Multicomponent Reactions. 2005:311–341.
- Bienayme H, Hulme C, Oddon G, Schmitt P. Chem Eur J. 2000;6(10):3321. - PubMed
-
- Dolle RE, La Bourdonnec B, Goodman AJ, Morales GA, Thomas CJ, Zhang W. J Comb Chem. 2009;11:739–790. - PubMed
- Dőmling A. Chem Rev. 2006;106:17–89. - PubMed
- Hulme C, Gore V. Curr Med Chem. 2003;10:51–80. - PubMed
- Hulme C, Lee YS. Mol Div. 2008;12:1–15. - PubMed
- Hulme C, Nixey T. Curr Opin Drug Discovery Dev. 2003;6:921–929. - PubMed
-
- Hulme C, Peng J, Tang SY, Burns CJ, Morize I, Labaudiniere R. J Org Chem. 1998;63:8021–8023.
- Nixey T, Hulme C. Tetrahedron Lett. 2002;43:6833–6835.
- Habashita H, Kokubo M, Hamano SI, Hamanaka N, Toda M, Shibayama S, Tada H, Sagawa K, Fukushima D, Maeda K, Mitsuya H. J Med Chem. 2006;49:4140–4152. - PubMed
- Nishizawa R, Nishiyama T, Hisaichi K, Matsunaga N, Minamoto C, Habashita H, Takaoka Y, Toda M, Shibayama S, Tada H, Sagawa K, Fukushima D, Maeda K, Mitsuya H. Bioorg Med Chem Lett. 2007;17:727–731. - PubMed
-
- Hulme C, Ma L, Romano J, Morton G, Tang S, Cherrier M, Choi S, Salvino J, Labaudiniere R. Tetrahedron Lett. 2000;41:1889–1893.
- Keating TA, Armstrong RW. J Org Chem. 1998;63:867–871. - PubMed
-
-
For the preparation of 4: 2-aminobenzyl-Cbz-amine (4a): To a solution of 2-aminobenzylamine (5.70 g, 46.7 mmol) in anhydrous dichloromethane (150 ml) was added DIPEA (16.30 ml, 93.0 mmol). Next, benzyl chloroformate (6.66 ml, 46.7 mmol) in anhydrous dichloromethane (45 ml) was added drop-wise via syringe. The reaction proceeded for 2 h. Then, reaction mixture was poured into a separatory funnel and washed with brine solution (3 x 80 ml). Organic layer was dried over MgSO4 and concentrated in vacuo to give yellowish white solid which was subsequently purified by Teledyne Isco CombiFlash Rf (Hexane/EtOAc 5–50%) to afford 4a (10.82g, 42.2 mmol, 90%) of yellowish white solid product. 1H NMR (300 MHz, CDCl3) δ 7.47 – 7.30 (m, 5H), 7.13 (td, J = 7.7, 1.6 Hz, 1H), 7.06 (dd, J = 7.4, 1.3 Hz, 1H), 6.65 – 6.75 (m, 2H), 5.15 (s, 2H), 5.08 (s, 1H), 4.33 (d, J = 6.2 Hz, 2H), 4.05 (s, 2H). 13C NMR (75 MHz, CDCl3) δ 157.34, 145.75, 136.74, 130.68, 129.63, 128.94, 128.59, 128.47, 122.53, 118.48, 116.33, 67.44, 42.92. [M+H]+ = 257. 2-Boc-aminobenzyl-Cbz-amine (4b): To a solution of 4a (10.74 g, 41.9 mmol) and DIPEA (14.64 ml, 84 mmol) in anhydrous dichloromethane (100 ml) was added Boc2O (10.97 g, 50.3 mmol). Reaction was then refluxed for 3 days. Reaction was then concentrated in vacuo and toluene was added to pull out t-BuOH giving 19.76 g light yellowish white solid which was then purified by Teledyne Isco CombiFlash Rf (Hexane/EtOAc 5–50%) to afford 4b (14.30 g, 40.1 mmol, 96%) of yellowish white solid product. 1H NMR (300 MHz, CDCl3) δ 7.86 (s, 1H), 7.82 (d, J = 8.0 Hz, 1H), 7.34 – 7.40 (d, J = 2.6 Hz, 5H), 7.31 (dd, J = 7.8, 1.7 Hz, 1H), 7.21 (dd, J = 7.5, 1.3 Hz, 1H), 7.06 (t, J = 7.4 Hz, 1H), 5.33 (s, 1H), 5.15 (s, 2H), 4.33 (d, J = 6.4 Hz, 2H), 1.55 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 157.58, 154.23, 137.14, 136.58, 130.47, 129.20, 128.95, 128.65, 128.56, 124.40, 123.22, 80.66, 67.60, 42.35, 28.77. [M+Na]+ = 379. 2-Boc-aminobenzylamine (4): To a solution of 4b (14.2 g, 39.8 mmol) in methanol (50 ml) was added 0.5 g Pd/C (10%). H2 (g) was flown into glass reactor at 40 psi. The reaction was then allowed to run at room temperature over night. Pd/C was removed using celite then solution was collected using vacuum filtration to yield 4 (8.80 g, 39.6 mmol, 99%) of yellow sticky product. 1H NMR (300 MHz, CDCl3) δ 9.48 (s, 1H), 7.99 (d, J = 8.1 Hz, 1H), 7.27 (td, J = 7.7, 1.6 Hz, 1H), 7.11 (dd, J = 7.4, 1.4 Hz, 1H), 6.96 (td, J = 7.4, 1.1 Hz, 1H), 3.97 (s, 2H), 1.74 (s, 2H), 1.54 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 153.86, 139.46, 129.37, 129.00, 128.66, 122.73, 120.72, 80.17, 45.91, 28.83. [M+H]+ = 223.
-
Grants and funding
LinkOut - more resources
Full Text Sources
